

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Serine protease 1 | ||
| Ligand | BDBM50112500 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEBML_212328 | ||
| Ki | 42±n/a nM | ||
| Citation |  Wu, S; Guilford, WJ; Chou, YL; Griedel, BD; Liang, A; Sakata, S; Shaw, KJ; Trinh, L; Xu, W; Zhao, Z; Morrissey, MM Design and synthesis of aminophenol-based factor Xa inhibitors. Bioorg Med Chem Lett12:1307-10 (2002) [PubMed] Wu, S; Guilford, WJ; Chou, YL; Griedel, BD; Liang, A; Sakata, S; Shaw, KJ; Trinh, L; Xu, W; Zhao, Z; Morrissey, MM Design and synthesis of aminophenol-based factor Xa inhibitors. Bioorg Med Chem Lett12:1307-10 (2002) [PubMed] | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Serine protease 1 | |||
| Name: | Serine protease 1 | ||
| Synonyms: | Beta-Trypsin | Cationic trypsin | PRSS1 | TRP1 | TRY1 | TRY1_BOVIN | TRYP1 | Trypsin | Trypsin I | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 25790.52 | ||
| Organism: | Bos taurus (bovine) | ||
| Description: | P00760 | ||
| Residue: | 246 | ||
| Sequence: |
| ||
| BDBM50112500 | |||
| n/a | |||
| Name | BDBM50112500 | ||
| Synonyms: | 3'-({4-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-nitro-phenylamino}-methyl)-biphenyl-3-carboxamidine | CHEMBL25461 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C27H30N6O3 | ||
| Mol. Mass. | 486.5655 | ||
| SMILES | CC([NH-])=[N+]1CCC(CC1)Oc1ccc(NCc2cccc(c2)-c2cccc(c2)C(N)=N)cc1[N+]([O-])=O |(-4.64,-1.92,;-3.23,-2.6,;-3.08,-4.13,;-2,-1.75,;-2.14,-.22,;-.91,.63,;.5,-.06,;.64,-1.56,;-.61,-2.43,;1.73,.79,;3.12,.12,;3.29,-1.4,;4.68,-2.08,;5.93,-1.21,;7.32,-1.89,;7.48,-3.42,;8.87,-4.09,;10.1,-3.21,;11.49,-3.88,;11.66,-5.42,;10.42,-6.28,;9.03,-5.61,;10.59,-7.79,;9.35,-8.66,;9.51,-10.19,;10.9,-10.86,;12.14,-10,;11.98,-8.47,;13.52,-10.71,;14.79,-9.91,;13.61,-12.25,;5.76,.32,;4.36,.98,;4.2,2.52,;5.43,3.37,;2.81,3.18,)| | ||
| Structure | 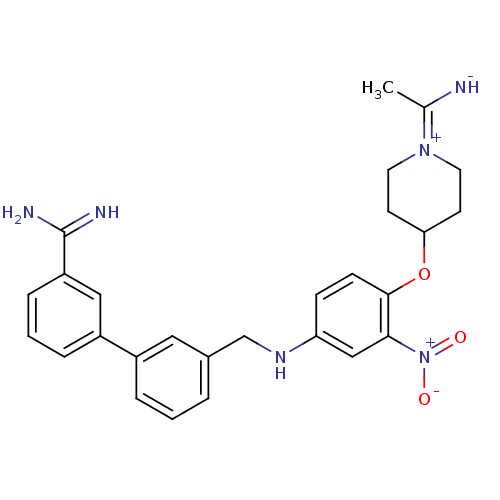
| ||