| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50553250 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2045812 (CHEMBL4700511) |
|---|
| IC50 | 17500±n/a nM |
|---|
| Citation |  Kirk, R; Ratcliffe, A; Noonan, G; Uosis-Martin, M; Lyth, D; Bardell-Cox, O; Massam, J; Schofield, P; Lyons, A; Clare, D; Maclean, J; Smith, A; Savage, V; Mohmed, S; Charrier, C; Salisbury, AM; Moyo, E; Ooi, N; Chalam-Judge, N; Cheung, J; Stokes, NR; Best, S; Craighead, M; Armer, R; Huxley, A Rational design, synthesis and testing of novel tricyclic topoisomerase inhibitors for the treatment of bacterial infections part 2. RSC Med Chem11:1379-1385 (2020) [PubMed] Article Kirk, R; Ratcliffe, A; Noonan, G; Uosis-Martin, M; Lyth, D; Bardell-Cox, O; Massam, J; Schofield, P; Lyons, A; Clare, D; Maclean, J; Smith, A; Savage, V; Mohmed, S; Charrier, C; Salisbury, AM; Moyo, E; Ooi, N; Chalam-Judge, N; Cheung, J; Stokes, NR; Best, S; Craighead, M; Armer, R; Huxley, A Rational design, synthesis and testing of novel tricyclic topoisomerase inhibitors for the treatment of bacterial infections part 2. RSC Med Chem11:1379-1385 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50553250 |
|---|
| n/a |
|---|
| Name | BDBM50553250 |
|---|
| Synonyms: | CHEMBL4786331 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H22ClFN4O2 |
|---|
| Mol. Mass. | 404.866 |
|---|
| SMILES | [H][C@]1(CCN(C1)c1c(F)cc2c3oc(C)nc3c(=O)n(C3CC3)c2c1Cl)[C@H](C)N |r| |
|---|
| Structure | 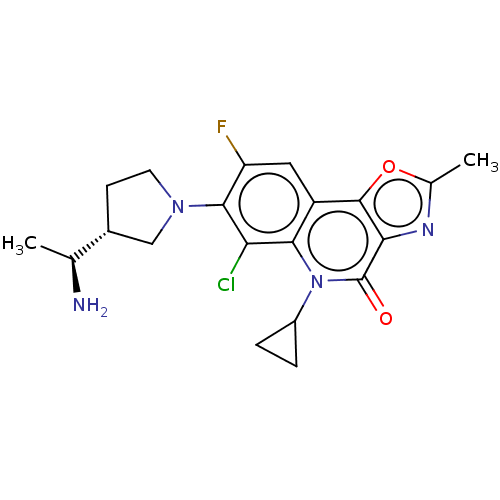
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kirk, R; Ratcliffe, A; Noonan, G; Uosis-Martin, M; Lyth, D; Bardell-Cox, O; Massam, J; Schofield, P; Lyons, A; Clare, D; Maclean, J; Smith, A; Savage, V; Mohmed, S; Charrier, C; Salisbury, AM; Moyo, E; Ooi, N; Chalam-Judge, N; Cheung, J; Stokes, NR; Best, S; Craighead, M; Armer, R; Huxley, A Rational design, synthesis and testing of novel tricyclic topoisomerase inhibitors for the treatment of bacterial infections part 2. RSC Med Chem11:1379-1385 (2020) [PubMed] Article
Kirk, R; Ratcliffe, A; Noonan, G; Uosis-Martin, M; Lyth, D; Bardell-Cox, O; Massam, J; Schofield, P; Lyons, A; Clare, D; Maclean, J; Smith, A; Savage, V; Mohmed, S; Charrier, C; Salisbury, AM; Moyo, E; Ooi, N; Chalam-Judge, N; Cheung, J; Stokes, NR; Best, S; Craighead, M; Armer, R; Huxley, A Rational design, synthesis and testing of novel tricyclic topoisomerase inhibitors for the treatment of bacterial infections part 2. RSC Med Chem11:1379-1385 (2020) [PubMed] Article