| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Ligand | BDBM50555743 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2053474 (CHEMBL4708475) |
|---|
| IC50 | 725±n/a nM |
|---|
| Citation |  Dinh, CP; Ville, A; Neukirch, K; Viault, G; Temml, V; Koeberle, A; Werz, O; Schuster, D; Stuppner, H; Richomme, P; Helesbeux, JJ; Séraphin, D Structure-based design, semi-synthesis and anti-inflammatory activity of tocotrienolic amides as 5-lipoxygenase inhibitors. Eur J Med Chem202:0 (2020) [PubMed] Article Dinh, CP; Ville, A; Neukirch, K; Viault, G; Temml, V; Koeberle, A; Werz, O; Schuster, D; Stuppner, H; Richomme, P; Helesbeux, JJ; Séraphin, D Structure-based design, semi-synthesis and anti-inflammatory activity of tocotrienolic amides as 5-lipoxygenase inhibitors. Eur J Med Chem202:0 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Name: | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Synonyms: | 5-LO | 5-Lipo-oxygenase (5-LOX) | 5-Lipoxygenase (5-LO) | 5-Lipoxygenase (LOX) | 5-Lipoygenase | 5-lipoxygenase/FLAP | ALOX5 | Arachidonate 5-lipoxygenase | LOG5 | LOX5_HUMAN |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 77972.74 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Recombinant protein was purified from E. coli lysate. After ammonium sulfate precipitation and subsequent steps, the supernatant (S100) was used for 5-LO activity assay.
|
|---|
| Residue: | 674 |
|---|
| Sequence: | MPSYTVTVATGSQWFAGTDDYIYLSLVGSAGCSEKHLLDKPFYNDFERGAVDSYDVTVDE
ELGEIQLVRIEKRKYWLNDDWYLKYITLKTPHGDYIEFPCYRWITGDVEVVLRDGRAKLA
RDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVL
NYSKAMENLFINRFMHMFQSSWNDFADFEKIFVKISNTISERVMNHWQEDLMFGYQFLNG
CNPVLIRRCTELPEKLPVTTEMVECSLERQLSLEQEVQQGNIFIVDFELLDGIDANKTDP
CTLQFLAAPICLLYKNLANKIVPIAIQLNQIPGDENPIFLPSDAKYDWLLAKIWVRSSDF
HVHQTITHLLRTHLVSEVFGIAMYRQLPAVHPIFKLLVAHVRFTIAINTKAREQLICECG
LFDKANATGGGGHVQMVQRAMKDLTYASLCFPEAIKARGMESKEDIPYYFYRDDGLLVWE
AIRTFTAEVVDIYYEGDQVVEEDPELQDFVNDVYVYGMRGRKSSGFPKSVKSREQLSEYL
TVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCW
HLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMARFRKNLEAIVSVIAERNKKKQLPY
YYLSPDRIPNSVAI
|
|
|
|---|
| BDBM50555743 |
|---|
| n/a |
|---|
| Name | BDBM50555743 |
|---|
| Synonyms: | CHEMBL4782136 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C37H51NO4 |
|---|
| Mol. Mass. | 573.8051 |
|---|
| SMILES | COc1cccc(CNC(=O)C(\C)=C\CC\C(C)=C\CC\C(C)=C\CC[C@]2(C)CCc3c(C)c(O)c(C)c(C)c3O2)c1 |r| |
|---|
| Structure | 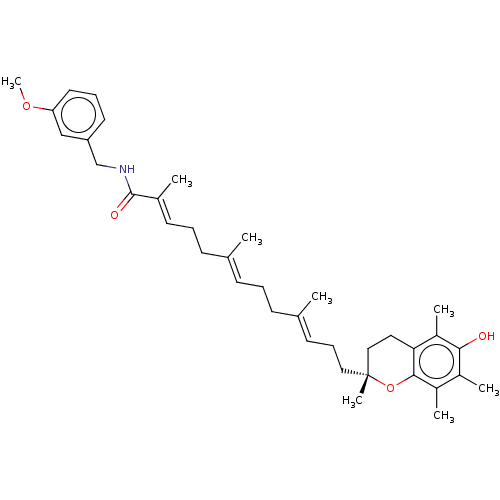
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Dinh, CP; Ville, A; Neukirch, K; Viault, G; Temml, V; Koeberle, A; Werz, O; Schuster, D; Stuppner, H; Richomme, P; Helesbeux, JJ; Séraphin, D Structure-based design, semi-synthesis and anti-inflammatory activity of tocotrienolic amides as 5-lipoxygenase inhibitors. Eur J Med Chem202:0 (2020) [PubMed] Article
Dinh, CP; Ville, A; Neukirch, K; Viault, G; Temml, V; Koeberle, A; Werz, O; Schuster, D; Stuppner, H; Richomme, P; Helesbeux, JJ; Séraphin, D Structure-based design, semi-synthesis and anti-inflammatory activity of tocotrienolic amides as 5-lipoxygenase inhibitors. Eur J Med Chem202:0 (2020) [PubMed] Article