| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A1 |
|---|
| Ligand | BDBM50053929 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_29633 (CHEMBL639740) |
|---|
| Ki | 305±n/a nM |
|---|
| Citation |  Okamura, T; Kurogi, Y; Nishikawa, H; Hashimoto, K; Fujiwara, H; Nagao, Y 1,2,4-Triazolo[5,1-i]purine derivatives as highly potent and selective human adenosine A(3) receptor ligands. J Med Chem45:3703-8 (2002) [PubMed] Okamura, T; Kurogi, Y; Nishikawa, H; Hashimoto, K; Fujiwara, H; Nagao, Y 1,2,4-Triazolo[5,1-i]purine derivatives as highly potent and selective human adenosine A(3) receptor ligands. J Med Chem45:3703-8 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A1 |
|---|
| Name: | Adenosine receptor A1 |
|---|
| Synonyms: | AA1R_RAT | ADENOSINE A1 | ADENOSINE A1 high | ADENOSINE A1 low | Adenosine A1 receptor (A1) | Adenosine receptor | Adenosine receptors A1 | Adora1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 36704.13 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | n/a |
|---|
| Residue: | 326 |
|---|
| Sequence: | MPPYISAFQAAYIGIEVLIALVSVPGNVLVIWAVKVNQALRDATFCFIVSLAVADVAVGA
LVIPLAILINIGPQTYFHTCLMVACPVLILTQSSILALLAIAVDRYLRVKIPLRYKTVVT
QRRAAVAIAGCWILSLVVGLTPMFGWNNLSVVEQDWRANGSVGEPVIKCEFEKVISMEYM
VYFNFFVWVLPPLLLMVLIYLEVFYLIRKQLNKKVSASSGDPQKYYGKELKIAKSLALIL
FLFALSWLPLHILNCITLFCPTCQKPSILIYIAIFLTHGNSAMNPIVYAFRIHKFRVTFL
KIWNDHFRCQPKPPIDEDLPEEKAED
|
|
|
|---|
| BDBM50053929 |
|---|
| n/a |
|---|
| Name | BDBM50053929 |
|---|
| Synonyms: | CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenyl-acetamide | N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-N-methyl-2-phenyl-acetamide | N-(9-chloro-2-(furan-2-yl)-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenylacetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H14ClN5O2 |
|---|
| Mol. Mass. | 403.821 |
|---|
| SMILES | Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 |
|---|
| Structure | 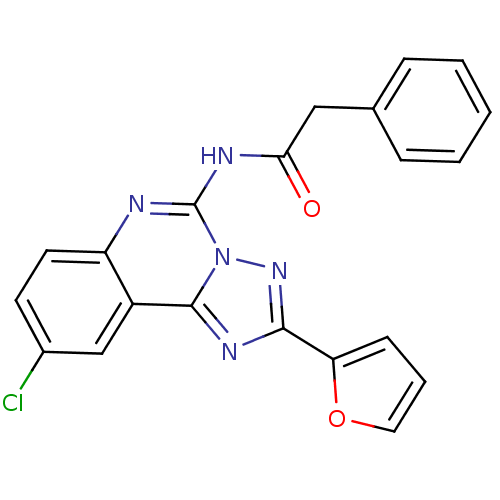
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Okamura, T; Kurogi, Y; Nishikawa, H; Hashimoto, K; Fujiwara, H; Nagao, Y 1,2,4-Triazolo[5,1-i]purine derivatives as highly potent and selective human adenosine A(3) receptor ligands. J Med Chem45:3703-8 (2002) [PubMed]
Okamura, T; Kurogi, Y; Nishikawa, H; Hashimoto, K; Fujiwara, H; Nagao, Y 1,2,4-Triazolo[5,1-i]purine derivatives as highly potent and selective human adenosine A(3) receptor ligands. J Med Chem45:3703-8 (2002) [PubMed]