| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor tyrosine-protein kinase erbB-4 |
|---|
| Ligand | BDBM50175583 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2070664 (CHEMBL4726198) |
|---|
| IC50 | 16±n/a nM |
|---|
| Citation |  de Bruin, G; Demont, D; de Zwart, E; Verkaik, S; Hoogenboom, N; van de Kar, B; van Lith, B; Emmelot-van Hoek, M; Gulrajani, M; Covey, T; Kaptein, A; Barf, T Discovery of quinoline-based irreversible BTK inhibitors. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article de Bruin, G; Demont, D; de Zwart, E; Verkaik, S; Hoogenboom, N; van de Kar, B; van Lith, B; Emmelot-van Hoek, M; Gulrajani, M; Covey, T; Kaptein, A; Barf, T Discovery of quinoline-based irreversible BTK inhibitors. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor tyrosine-protein kinase erbB-4 |
|---|
| Name: | Receptor tyrosine-protein kinase erbB-4 |
|---|
| Synonyms: | ERBB4 | ERBB4_HUMAN | Epidermal growth factor receptor | Epidermal growth factor receptor 4 (ERBB4) | ErbB-4 (HER4) Tyrosine Kinase | HER4 | Proto-oncogene c-ErbB-4 | Proto-oncogene-like protein c-ErbB-4 | Receptor protein-tyrosine kinase erbB-4 | Tyrosine kinase-type cell surface receptor HER4 | Tyrosine kinase-type cell surface receptor HER4 (HER4) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 146803.56 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q15303 |
|---|
| Residue: | 1308 |
|---|
| Sequence: | MKPATGLWVWVSLLVAAGTVQPSDSQSVCAGTENKLSSLSDLEQQYRALRKYYENCEVVM
GNLEITSIEHNRDLSFLRSVREVTGYVLVALNQFRYLPLENLRIIRGTKLYEDRYALAIF
LNYRKDGNFGLQELGLKNLTEILNGGVYVDQNKFLCYADTIHWQDIVRNPWPSNLTLVST
NGSSGCGRCHKSCTGRCWGPTENHCQTLTRTVCAEQCDGRCYGPYVSDCCHRECAGGCSG
PKDTDCFACMNFNDSGACVTQCPQTFVYNPTTFQLEHNFNAKYTYGAFCVKKCPHNFVVD
SSSCVRACPSSKMEVEENGIKMCKPCTDICPKACDGIGTGSLMSAQTVDSSNIDKFINCT
KINGNLIFLVTGIHGDPYNAIEAIDPEKLNVFRTVREITGFLNIQSWPPNMTDFSVFSNL
VTIGGRVLYSGLSLLILKQQGITSLQFQSLKEISAGNIYITDNSNLCYYHTINWTTLFST
INQRIVIRDNRKAENCTAEGMVCNHLCSSDGCWGPGPDQCLSCRRFSRGRICIESCNLYD
GEFREFENGSICVECDPQCEKMEDGLLTCHGPGPDNCTKCSHFKDGPNCVEKCPDGLQGA
NSFIFKYADPDRECHPCHPNCTQGCNGPTSHDCIYYPWTGHSTLPQHARTPLIAAGVIGG
LFILVIVGLTFAVYVRRKSIKKKRALRRFLETELVEPLTPSGTAPNQAQLRILKETELKR
VKVLGSGAFGTVYKGIWVPEGETVKIPVAIKILNETTGPKANVEFMDEALIMASMDHPHL
VRLLGVCLSPTIQLVTQLMPHGCLLEYVHEHKDNIGSQLLLNWCVQIAKGMMYLEERRLV
HRDLAARNVLVKSPNHVKITDFGLARLLEGDEKEYNADGGKMPIKWMALECIHYRKFTHQ
SDVWSYGVTIWELMTFGGKPYDGIPTREIPDLLEKGERLPQPPICTIDVYMVMVKCWMID
ADSRPKFKELAAEFSRMARDPQRYLVIQGDDRMKLPSPNDSKFFQNLLDEEDLEDMMDAE
EYLVPQAFNIPPPIYTSRARIDSNRSEIGHSPPPAYTPMSGNQFVYRDGGFAAEQGVSVP
YRAPTSTIPEAPVAQGATAEIFDDSCCNGTLRKPVAPHVQEDSSTQRYSADPTVFAPERS
PRGELDEEGYMTPMRDKPKQEYLNPVEENPFVSRRKNGDLQALDNPEYHNASNGPPKAED
EYVNEPLYLNTFANTLGKAEYLKNNILSMPEKAKKAFDNPDYWNHSLPPRSTLQHPDYLQ
EYSTKYFYKQNGRIRPIVAENPEYLSEFSLKPGTVLPPPPYRHRNTVV
|
|
|
|---|
| BDBM50175583 |
|---|
| n/a |
|---|
| Name | BDBM50175583 |
|---|
| Synonyms: | ACP-196 | Acalabrutinib | US10239883, Example 6 | US10793576, Example ACP-196 | US10919899, ACP-196 | US10934296, Example 6 | US11420975, Acalabrutinlb | US9758524, Example 6 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H23N7O2 |
|---|
| Mol. Mass. | 465.5065 |
|---|
| SMILES | [H][C@]1(CCCN1C(=O)C#CC)c1nc(-c2ccc(cc2)C(=O)Nc2ccccn2)c2c(N)nccn12 |r| |
|---|
| Structure | 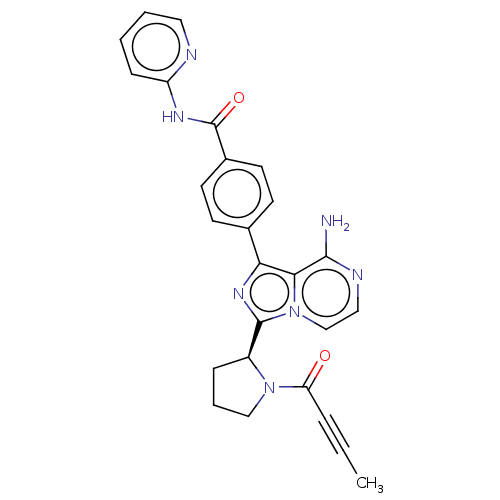
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 de Bruin, G; Demont, D; de Zwart, E; Verkaik, S; Hoogenboom, N; van de Kar, B; van Lith, B; Emmelot-van Hoek, M; Gulrajani, M; Covey, T; Kaptein, A; Barf, T Discovery of quinoline-based irreversible BTK inhibitors. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article
de Bruin, G; Demont, D; de Zwart, E; Verkaik, S; Hoogenboom, N; van de Kar, B; van Lith, B; Emmelot-van Hoek, M; Gulrajani, M; Covey, T; Kaptein, A; Barf, T Discovery of quinoline-based irreversible BTK inhibitors. Bioorg Med Chem Lett30:0 (2020) [PubMed] Article