| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-2A adrenergic receptor |
|---|
| Ligand | BDBM50131368 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_33076 |
|---|
| Ki | 98±n/a nM |
|---|
| Citation |  Andrés, JI; Alcázar, J; Alonso, JM; Alvarez, RM; Cid, JM; De Lucas, AI; Fernández, J; Martínez, S; Nieto, C; Pastor, J; Bakker, MH; Biesmans, I; Heylen, LI; Megens, AA Synthesis of 3a,4-dihydro-3H-[1]benzopyrano[4,3-c]isoxazoles, displaying combined 5-HT uptake inhibiting and alpha(2)-adrenoceptor antagonistic activities: a novel series of potential antidepressants. Bioorg Med Chem Lett13:2719-25 (2003) [PubMed] Andrés, JI; Alcázar, J; Alonso, JM; Alvarez, RM; Cid, JM; De Lucas, AI; Fernández, J; Martínez, S; Nieto, C; Pastor, J; Bakker, MH; Biesmans, I; Heylen, LI; Megens, AA Synthesis of 3a,4-dihydro-3H-[1]benzopyrano[4,3-c]isoxazoles, displaying combined 5-HT uptake inhibiting and alpha(2)-adrenoceptor antagonistic activities: a novel series of potential antidepressants. Bioorg Med Chem Lett13:2719-25 (2003) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-2A adrenergic receptor |
|---|
| Name: | Alpha-2A adrenergic receptor |
|---|
| Synonyms: | ADA2A_HUMAN | ADRA2A | ADRA2R | ADRAR | Adrenergic alpha2A | Adrenergic receptor alpha | Alpha-2 adrenergic receptor subtype C10 | Alpha-2A adrenoceptor | Alpha-2A adrenoreceptor | Alpha-2AAR | alpha-2A adrenergic receptor [Homo sapiens] |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48979.91 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08913 |
|---|
| Residue: | 465 |
|---|
| Sequence: | MFRQEQPLAEGSFAPMGSLQPDAGNASWNGTEAPGGGARATPYSLQVTLTLVCLAGLLML
LTVFGNVLVIIAVFTSRALKAPQNLFLVSLASADILVATLVIPFSLANEVMGYWYFGKAW
CEIYLALDVLFCTSSIVHLCAISLDRYWSITQAIEYNLKRTPRRIKAIIITVWVISAVIS
FPPLISIEKKGGGGGPQPAEPRCEINDQKWYVISSCIGSFFAPCLIMILVYVRIYQIAKR
RTRVPPSRRGPDAVAAPPGGTERRPNGLGPERSAGPGGAEAEPLPTQLNGAPGEPAPAGP
RDTDALDLEESSSSDHAERPPGPRRPERGPRGKGKARASQVKPGDSLPRRGPGATGIGTP
AAGPGEERVGAAKASRWRGRQNREKRFTFVLAVVIGVFVVCWFPFFFTYTLTAVGCSVPR
TLFKFFFWFGYCNSSLNPVIYTIFNHDFRRAFKKILCRGDRKRIV
|
|
|
|---|
| BDBM50131368 |
|---|
| n/a |
|---|
| Name | BDBM50131368 |
|---|
| Synonyms: | 3-(4-Benzo[b]thiophen-2-ylmethyl-piperazin-1-ylmethyl)-7,8-dimethoxy-3a,4-dihydro-3H-chromeno[4,3-c]isoxazole | CHEMBL94435 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H29N3O4S |
|---|
| Mol. Mass. | 479.591 |
|---|
| SMILES | COc1cc2OCC3C(CN4CCN(Cc5cc6ccccc6s5)CC4)ON=C3c2cc1OC |c:30| |
|---|
| Structure | 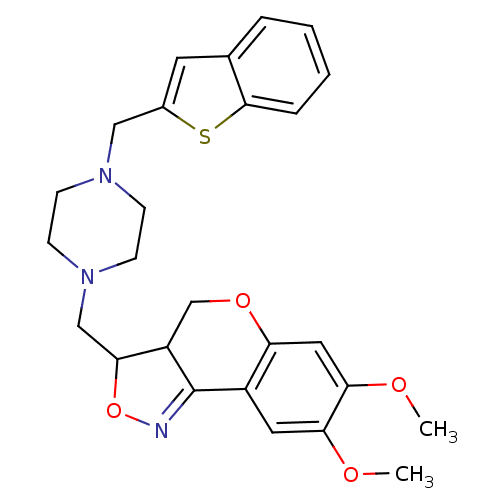
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Andrés, JI; Alcázar, J; Alonso, JM; Alvarez, RM; Cid, JM; De Lucas, AI; Fernández, J; Martínez, S; Nieto, C; Pastor, J; Bakker, MH; Biesmans, I; Heylen, LI; Megens, AA Synthesis of 3a,4-dihydro-3H-[1]benzopyrano[4,3-c]isoxazoles, displaying combined 5-HT uptake inhibiting and alpha(2)-adrenoceptor antagonistic activities: a novel series of potential antidepressants. Bioorg Med Chem Lett13:2719-25 (2003) [PubMed]
Andrés, JI; Alcázar, J; Alonso, JM; Alvarez, RM; Cid, JM; De Lucas, AI; Fernández, J; Martínez, S; Nieto, C; Pastor, J; Bakker, MH; Biesmans, I; Heylen, LI; Megens, AA Synthesis of 3a,4-dihydro-3H-[1]benzopyrano[4,3-c]isoxazoles, displaying combined 5-HT uptake inhibiting and alpha(2)-adrenoceptor antagonistic activities: a novel series of potential antidepressants. Bioorg Med Chem Lett13:2719-25 (2003) [PubMed]