

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Dipeptidyl peptidase 4 | ||
| Ligand | BDBM50137266 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_304955 (CHEMBL827834) | ||
| IC50 | 2.6±n/a nM | ||
| Citation |  Weber, AE Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem47:4135-41 (2004) [PubMed] Article Weber, AE Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem47:4135-41 (2004) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Dipeptidyl peptidase 4 | |||
| Name: | Dipeptidyl peptidase 4 | ||
| Synonyms: | ADABP | ADCP2 | Adenosine deaminase complexing protein 2 | CD26 | CD_antigen=CD26 | DPP IV | DPP4 | DPP4_HUMAN | DPPIV | Dipeptidyl peptidase 4 (DDP-IV) | Dipeptidyl peptidase 4 (DPP IV) | Dipeptidyl peptidase 4 (DPP-4) | Dipeptidyl peptidase 4 (DPP4) | Dipeptidyl peptidase 4 (DPPIV) | Dipeptidyl peptidase 4 membrane form | Dipeptidyl peptidase 4 soluble form | Dipeptidyl peptidase IV | Dipeptidyl peptidase IV (DDP-4) | Dipeptidyl peptidase IV (DDP-IV) | Dipeptidyl peptidase IV (DPP IV) | Dipeptidyl peptidase IV membrane form | Dipeptidyl peptidase IV soluble form | Dipeptidyl peptidase-IV (DPP-4) | Dipeptidyl peptidase-IV (DPP-IV) | T-cell activation antigen CD26 | TP103 | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 88271.01 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P27487 | ||
| Residue: | 766 | ||
| Sequence: |
| ||
| BDBM50137266 | |||
| n/a | |||
| Name | BDBM50137266 | ||
| Synonyms: | CHEMBL140577 | N-[4-((S)-1-Amino-2-oxo-2-pyrrolidin-1-yl-ethyl)-cyclohexyl]-4-(2,2,2-trifluoro-ethanesulfonylamino)-benzenesulfonamide | N-[4-(1-Amino-2-oxo-2-pyrrolidin-1-yl-ethyl)-cyclohexyl]-4-(2,2,2-trifluoro-ethanesulfonylamino)-benzenesulfonamide | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H29F3N4O5S2 | ||
| Mol. Mass. | 526.593 | ||
| SMILES | N[C@@H](C1CC[C@@H](CC1)NS(=O)(=O)c1ccc(NS(=O)(=O)CC(F)(F)F)cc1)C(=O)N1CCCC1 |wU:5.8,wD:1.0,(10.78,8.99,;12.11,8.21,;12.11,6.69,;10.79,5.9,;10.79,4.36,;12.11,3.59,;13.44,4.36,;13.44,5.9,;12.11,2.05,;13.45,1.28,;13.45,-.26,;14.78,2.05,;14.78,.51,;14.76,-1.01,;16.09,-1.78,;17.42,-1.03,;18.76,-1.8,;20.09,-1.03,;20.09,.51,;21.43,-1.8,;21.42,-.24,;22.76,-1,;23.98,-1.69,;23.03,-2.59,;24.26,-.4,;17.42,.53,;16.09,1.28,;13.45,8.99,;13.45,10.53,;14.78,8.22,;16.18,8.83,;17.22,7.7,;16.45,6.36,;14.94,6.69,)| | ||
| Structure | 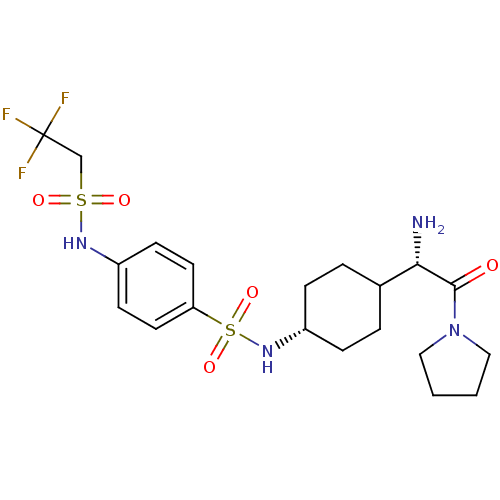
| ||