| Reaction Details |
|---|
 | Report a problem with these data |
| Target | alpha-1,2-Mannosidase |
|---|
| Ligand | BDBM50169002 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_305169 (CHEMBL832759) |
|---|
| IC50 | 110000±n/a nM |
|---|
| Citation |  Fiaux, H; Popowycz, F; Favre, S; Schütz, C; Vogel, P; Gerber-Lemaire, S; Juillerat-Jeanneret, L Functionalized pyrrolidines inhibit alpha-mannosidase activity and growth of human glioblastoma and melanoma cells. J Med Chem48:4237-46 (2005) [PubMed] Article Fiaux, H; Popowycz, F; Favre, S; Schütz, C; Vogel, P; Gerber-Lemaire, S; Juillerat-Jeanneret, L Functionalized pyrrolidines inhibit alpha-mannosidase activity and growth of human glioblastoma and melanoma cells. J Med Chem48:4237-46 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| alpha-1,2-Mannosidase |
|---|
| Name: | alpha-1,2-Mannosidase |
|---|
| Synonyms: | Alpha-mannosidase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65344.50 |
|---|
| Organism: | Glycine max |
|---|
| Description: | ChEMBL_32380 |
|---|
| Residue: | 578 |
|---|
| Sequence: | MARGSRSVGSSSSKWRYCNPSYYLKRPKRLALLFIVFVCVSFVFWDRQTLVREHQVEISE
LQKEVTDLKNLVDDLNNKQGGTSGKTDLGRKATKSSKDVLDDPIDIERREKVKEAMLHAW
GSYEKYAWGQDELQPQSKNGVNSFGGLGATLIDSLDTLYIMGLNEQFQKAREWVANSLDF
NKDYEASVFETTIRVVGGLLSAYDLSGDKVFLDKAIEIADRLLPAWNTPTGIPYNIINLS
HGRAHNPSWTGGESILADSGTEQLEFIVLSQRTGDLKYQQKVENVIAQLNKTFPDDGLLP
IYINPHSGAAGYSPITFGAMGDSFYEYLLKVWIQGNKTSSIKHYRDMWEKSMKGLSSLIR
RSTPSSFTYICEKNGGSLTDKMDELACFAPGMIALGSFGYSAADDSQKFLSLAEELAWTC
YNFYQSTPTKLAGENYFFHSGQDMSVGTSWNILRPETVESLFYLWRLTGNKTYQEWGWNI
FQAFEKNSRIESGYVGLKDVNSGVKDNMMQSFFLAETLKYFYLLFSPSSVISLDEWVFNT
EAHPLRIVTRHEEGLVKNLNEKQKPFSRIGGRKEGRSG
|
|
|
|---|
| BDBM50169002 |
|---|
| n/a |
|---|
| Name | BDBM50169002 |
|---|
| Synonyms: | (2R,3R,4S)-2-[((S)-Phenyl-1-(R)-2-hydroxy-2-phenyl-ethylamino)-methyl]-pyrrolidine-3,4-diol | CHEMBL188227 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H24N2O3 |
|---|
| Mol. Mass. | 328.4055 |
|---|
| SMILES | O[C@H]([C@H](NC[C@H]1NC[C@H](O)[C@@H]1O)c1ccccc1)c1ccccc1 |
|---|
| Structure | 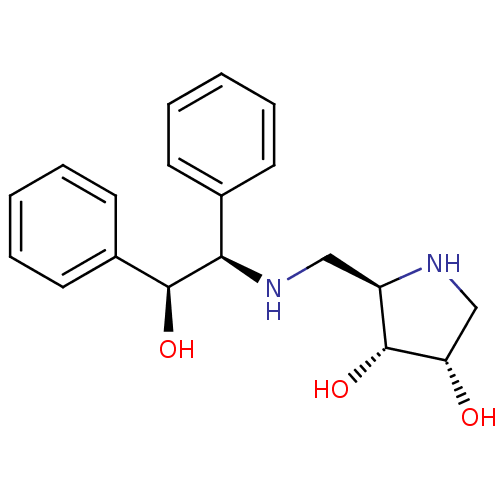
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Fiaux, H; Popowycz, F; Favre, S; Schütz, C; Vogel, P; Gerber-Lemaire, S; Juillerat-Jeanneret, L Functionalized pyrrolidines inhibit alpha-mannosidase activity and growth of human glioblastoma and melanoma cells. J Med Chem48:4237-46 (2005) [PubMed] Article
Fiaux, H; Popowycz, F; Favre, S; Schütz, C; Vogel, P; Gerber-Lemaire, S; Juillerat-Jeanneret, L Functionalized pyrrolidines inhibit alpha-mannosidase activity and growth of human glioblastoma and melanoma cells. J Med Chem48:4237-46 (2005) [PubMed] Article