| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50172123 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_321392 (CHEMBL880627) |
|---|
| IC50 | 608±n/a nM |
|---|
| Citation |  Chua, PC; Nagasawa, JY; Bleicher, LS; Munoz, B; Schweiger, EJ; Tehrani, L; Anderson, JJ; Cramer, M; Chung, J; Green, MD; King, CD; Reyes-Manalo, G; Cosford, ND Cyclohexenyl- and dehydropiperidinyl-alkynyl pyridines as potent metabotropic glutamate subtype 5 (mGlu5) receptor antagonists. Bioorg Med Chem Lett15:4589-93 (2005) [PubMed] Article Chua, PC; Nagasawa, JY; Bleicher, LS; Munoz, B; Schweiger, EJ; Tehrani, L; Anderson, JJ; Cramer, M; Chung, J; Green, MD; King, CD; Reyes-Manalo, G; Cosford, ND Cyclohexenyl- and dehydropiperidinyl-alkynyl pyridines as potent metabotropic glutamate subtype 5 (mGlu5) receptor antagonists. Bioorg Med Chem Lett15:4589-93 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50172123 |
|---|
| n/a |
|---|
| Name | BDBM50172123 |
|---|
| Synonyms: | 3-Chloro-5-(3-pyridin-2-ylethynyl-cyclohex-2-enyloxy)-pyridine | CHEMBL381192 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H15ClN2O |
|---|
| Mol. Mass. | 310.778 |
|---|
| SMILES | Clc1cncc(OC2CCCC(=C2)C#Cc2ccccn2)c1 |c:11| |
|---|
| Structure | 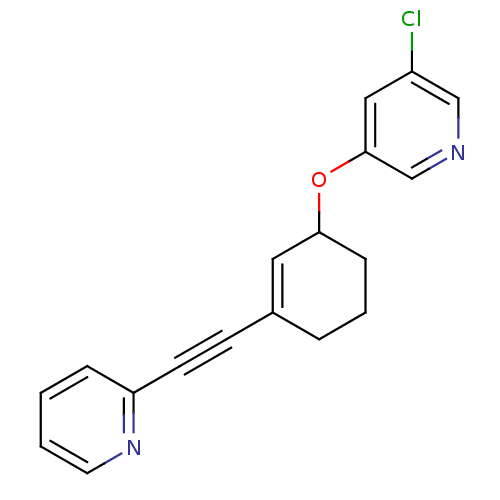
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chua, PC; Nagasawa, JY; Bleicher, LS; Munoz, B; Schweiger, EJ; Tehrani, L; Anderson, JJ; Cramer, M; Chung, J; Green, MD; King, CD; Reyes-Manalo, G; Cosford, ND Cyclohexenyl- and dehydropiperidinyl-alkynyl pyridines as potent metabotropic glutamate subtype 5 (mGlu5) receptor antagonists. Bioorg Med Chem Lett15:4589-93 (2005) [PubMed] Article
Chua, PC; Nagasawa, JY; Bleicher, LS; Munoz, B; Schweiger, EJ; Tehrani, L; Anderson, JJ; Cramer, M; Chung, J; Green, MD; King, CD; Reyes-Manalo, G; Cosford, ND Cyclohexenyl- and dehydropiperidinyl-alkynyl pyridines as potent metabotropic glutamate subtype 5 (mGlu5) receptor antagonists. Bioorg Med Chem Lett15:4589-93 (2005) [PubMed] Article