| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2B6 |
|---|
| Ligand | BDBM50604138 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2246999 (CHEMBL5161209) |
|---|
| IC50 | >20000±n/a nM |
|---|
| Citation |  Luo, G; Chen, L; Kostich, WA; Hamman, B; Allen, J; Easton, A; Bourin, C; Gulianello, M; Lippy, J; Nara, S; Pattipati, SN; Dandapani, K; Dokania, M; Vattikundala, P; Sharma, V; Elavazhagan, S; Verma, MK; Lal Das, M; Wagh, S; Balakrishnan, A; Johnson, BM; Santone, KS; Thalody, G; Denton, R; Saminathan, H; Holenarsipur, VK; Kumar, A; Rao, A; Putlur, SP; Sarvasiddhi, SK; Shankar, G; Louis, JV; Ramarao, M; Conway, CM; Li, YW; Pieschl, R; Tian, Y; Hong, Y; Bristow, L; Albright, CF; Bronson, JJ; Macor, JE; Dzierba, CD Discovery and Optimization of Biaryl Alkyl Ethers as a Novel Class of Highly Selective, CNS-Penetrable, and Orally Active Adaptor Protein-2-Associated Kinase 1 (AAK1) Inhibitors for the Potential Treatment of Neuropathic Pain. J Med Chem65:4534-4564 (2022) [PubMed] Article Luo, G; Chen, L; Kostich, WA; Hamman, B; Allen, J; Easton, A; Bourin, C; Gulianello, M; Lippy, J; Nara, S; Pattipati, SN; Dandapani, K; Dokania, M; Vattikundala, P; Sharma, V; Elavazhagan, S; Verma, MK; Lal Das, M; Wagh, S; Balakrishnan, A; Johnson, BM; Santone, KS; Thalody, G; Denton, R; Saminathan, H; Holenarsipur, VK; Kumar, A; Rao, A; Putlur, SP; Sarvasiddhi, SK; Shankar, G; Louis, JV; Ramarao, M; Conway, CM; Li, YW; Pieschl, R; Tian, Y; Hong, Y; Bristow, L; Albright, CF; Bronson, JJ; Macor, JE; Dzierba, CD Discovery and Optimization of Biaryl Alkyl Ethers as a Novel Class of Highly Selective, CNS-Penetrable, and Orally Active Adaptor Protein-2-Associated Kinase 1 (AAK1) Inhibitors for the Potential Treatment of Neuropathic Pain. J Med Chem65:4534-4564 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2B6 |
|---|
| Name: | Cytochrome P450 2B6 |
|---|
| Synonyms: | CP2B6_HUMAN | CYP2B6 | Cytochrome P450 2B6 (CYP2B6) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56289.75 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P20813 |
|---|
| Residue: | 491 |
|---|
| Sequence: | MELSVLLFLALLTGLLLLLVQRHPNTHDRLPPGPRPLPLLGNLLQMDRRGLLKSFLRFRE
KYGDVFTVHLGPRPVVMLCGVEAIREALVDKAEAFSGRGKIAMVDPFFRGYGVIFANGNR
WKVLRRFSVTTMRDFGMGKRSVEERIQEEAQCLIEELRKSKGALMDPTFLFQSITANIIC
SIVFGKRFHYQDQEFLKMLNLFYQTFSLISSVFGQLFELFSGFLKYFPGAHRQVYKNLQE
INAYIGHSVEKHRETLDPSAPKDLIDTYLLHMEKEKSNAHSEFSHQNLNLNTLSLFFAGT
ETTSTTLRYGFLLMLKYPHVAERVYREIEQVIGPHRPPELHDRAKMPYTEAVIYEIQRFS
DLLPMGVPHIVTQHTSFRGYIIPKDTEVFLILSTALHDPHYFEKPDAFNPDHFLDANGAL
KKTEAFIPFSLGKRICLGEGIARAELFLFFTTILQNFSMASPVAPEDIDLTPQECGVGKI
PPTYQIRFLPR
|
|
|
|---|
| BDBM50604138 |
|---|
| n/a |
|---|
| Name | BDBM50604138 |
|---|
| Synonyms: | CHEMBL5184381 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H28N4O3 |
|---|
| Mol. Mass. | 372.4613 |
|---|
| SMILES | COC(=O)Nc1cc(ccn1)-c1cnc(OC[C@@](C)(N)CC(C)C)c(C)c1 |r| |
|---|
| Structure | 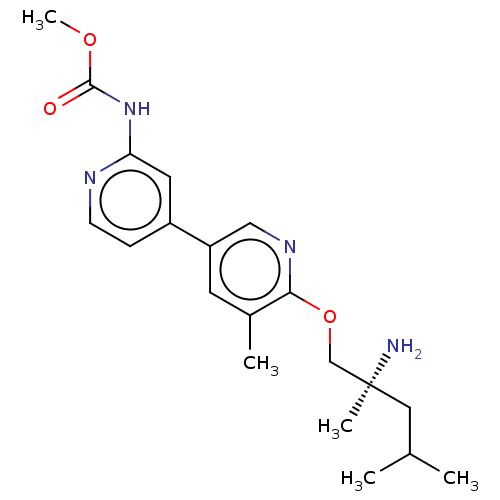
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Luo, G; Chen, L; Kostich, WA; Hamman, B; Allen, J; Easton, A; Bourin, C; Gulianello, M; Lippy, J; Nara, S; Pattipati, SN; Dandapani, K; Dokania, M; Vattikundala, P; Sharma, V; Elavazhagan, S; Verma, MK; Lal Das, M; Wagh, S; Balakrishnan, A; Johnson, BM; Santone, KS; Thalody, G; Denton, R; Saminathan, H; Holenarsipur, VK; Kumar, A; Rao, A; Putlur, SP; Sarvasiddhi, SK; Shankar, G; Louis, JV; Ramarao, M; Conway, CM; Li, YW; Pieschl, R; Tian, Y; Hong, Y; Bristow, L; Albright, CF; Bronson, JJ; Macor, JE; Dzierba, CD Discovery and Optimization of Biaryl Alkyl Ethers as a Novel Class of Highly Selective, CNS-Penetrable, and Orally Active Adaptor Protein-2-Associated Kinase 1 (AAK1) Inhibitors for the Potential Treatment of Neuropathic Pain. J Med Chem65:4534-4564 (2022) [PubMed] Article
Luo, G; Chen, L; Kostich, WA; Hamman, B; Allen, J; Easton, A; Bourin, C; Gulianello, M; Lippy, J; Nara, S; Pattipati, SN; Dandapani, K; Dokania, M; Vattikundala, P; Sharma, V; Elavazhagan, S; Verma, MK; Lal Das, M; Wagh, S; Balakrishnan, A; Johnson, BM; Santone, KS; Thalody, G; Denton, R; Saminathan, H; Holenarsipur, VK; Kumar, A; Rao, A; Putlur, SP; Sarvasiddhi, SK; Shankar, G; Louis, JV; Ramarao, M; Conway, CM; Li, YW; Pieschl, R; Tian, Y; Hong, Y; Bristow, L; Albright, CF; Bronson, JJ; Macor, JE; Dzierba, CD Discovery and Optimization of Biaryl Alkyl Ethers as a Novel Class of Highly Selective, CNS-Penetrable, and Orally Active Adaptor Protein-2-Associated Kinase 1 (AAK1) Inhibitors for the Potential Treatment of Neuropathic Pain. J Med Chem65:4534-4564 (2022) [PubMed] Article