| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50275963 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_492785 (CHEMBL939473) |
|---|
| IC50 | 15±n/a nM |
|---|
| Citation |  Moorjani, M; Luo, Z; Lin, E; Vong, BG; Chen, Y; Zhang, X; Rueter, JK; Gross, RS; Lanier, MC; Tellew, JE; Williams, JP; Lechner, SM; Malany, S; Santos, M; Crespo, MI; Díaz, JL; Saunders, J; Slee, DH 2,6-Diaryl-4-acylaminopyrimidines as potent and selective adenosine A(2A) antagonists with improved solubility and metabolic stability. Bioorg Med Chem Lett18:5402-5 (2008) [PubMed] Article Moorjani, M; Luo, Z; Lin, E; Vong, BG; Chen, Y; Zhang, X; Rueter, JK; Gross, RS; Lanier, MC; Tellew, JE; Williams, JP; Lechner, SM; Malany, S; Santos, M; Crespo, MI; Díaz, JL; Saunders, J; Slee, DH 2,6-Diaryl-4-acylaminopyrimidines as potent and selective adenosine A(2A) antagonists with improved solubility and metabolic stability. Bioorg Med Chem Lett18:5402-5 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | AA2AR_RAT | ADENOSINE A2a | Adenosine A2 receptor | Adenosine A2a receptor (A2a) | Adenosine Receptors A2a (A2a) | Adenosine receptor A2a and A3 | Adenosine receptors A2a | Adora2a | Rat striatal adenosine A2a receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 45015.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat A2A receptors expressed in CHO cells. |
|---|
| Residue: | 410 |
|---|
| Sequence: | MGSSVYITVELAIAVLAILGNVLVCWAVWINSNLQNVTNFFVVSLAAADIAVGVLAIPFA
ITISTGFCAACHGCLFFACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGVRAKG
IIAICWVLSFAIGLTPMLGWNNCSQKDGNSTKTCGEGRVTCLFEDVVPMNYMVYYNFFAF
VLLPLLLMLAIYLRIFLAARRQLKQMESQPLPGERTRSTLQKEVHAAKSLAIIVGLFALC
WLPLHIINCFTFFCSTCRHAPPWLMYLAIILSHSNSVVNPFIYAYRIREFRQTFRKIIRT
HVLRRQEPFQAGGSSAWALAAHSTEGEQVSLRLNGHPLGVWANGSATHSGRRPNGYTLGL
GGGGSAQGSPRDVELPTQERQEGQEHPGLRGHLVQARVGASSWSSEFAPS
|
|
|
|---|
| BDBM50275963 |
|---|
| n/a |
|---|
| Name | BDBM50275963 |
|---|
| Synonyms: | (S)-N-(2-(3,5-dimethyl-1H-pyrazol-1-yl)-6-(3-fluoro-5-(1-methylpyrrolidin-3-yloxy)phenyl)pyrimidin-4-yl)acetamide | CHEMBL511440 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H25FN6O2 |
|---|
| Mol. Mass. | 424.4713 |
|---|
| SMILES | CN1CC[C@@H](C1)Oc1cc(F)cc(c1)-c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C |r| |
|---|
| Structure | 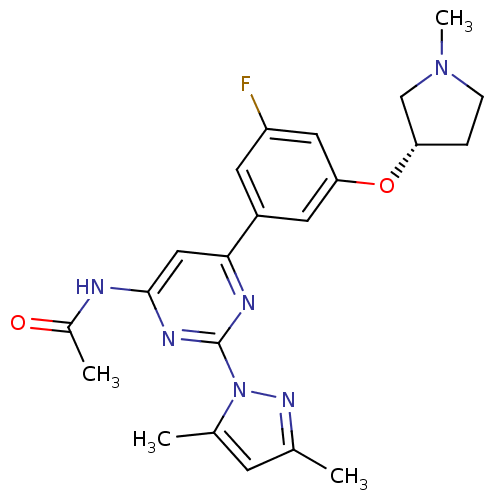
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Moorjani, M; Luo, Z; Lin, E; Vong, BG; Chen, Y; Zhang, X; Rueter, JK; Gross, RS; Lanier, MC; Tellew, JE; Williams, JP; Lechner, SM; Malany, S; Santos, M; Crespo, MI; Díaz, JL; Saunders, J; Slee, DH 2,6-Diaryl-4-acylaminopyrimidines as potent and selective adenosine A(2A) antagonists with improved solubility and metabolic stability. Bioorg Med Chem Lett18:5402-5 (2008) [PubMed] Article
Moorjani, M; Luo, Z; Lin, E; Vong, BG; Chen, Y; Zhang, X; Rueter, JK; Gross, RS; Lanier, MC; Tellew, JE; Williams, JP; Lechner, SM; Malany, S; Santos, M; Crespo, MI; Díaz, JL; Saunders, J; Slee, DH 2,6-Diaryl-4-acylaminopyrimidines as potent and selective adenosine A(2A) antagonists with improved solubility and metabolic stability. Bioorg Med Chem Lett18:5402-5 (2008) [PubMed] Article