| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Equilibrative nucleoside transporter 1 |
|---|
| Ligand | BDBM23617 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_518728 (CHEMBL957103) |
|---|
| Ki | 0.7±n/a nM |
|---|
| Citation |  Gupte, A; Buolamwini, JK Synthesis and biological evaluation of phloridzin analogs as human concentrative nucleoside transporter 3 (hCNT3) inhibitors. Bioorg Med Chem Lett19:917-21 (2009) [PubMed] Article Gupte, A; Buolamwini, JK Synthesis and biological evaluation of phloridzin analogs as human concentrative nucleoside transporter 3 (hCNT3) inhibitors. Bioorg Med Chem Lett19:917-21 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Equilibrative nucleoside transporter 1 |
|---|
| Name: | Equilibrative nucleoside transporter 1 |
|---|
| Synonyms: | ENT1 | Equilibrative NBMPR-sensitive nucleoside transporter | Equilibrative Nucleoside Transporter 1 (ENT1) | Equilibrative nitrobenzylmercaptopurine riboside-sensitive nucleoside transporter | Equilibrative nucleoside transporter 1 | Nucleoside transporter, es-type | S29A1_HUMAN | SLC29A1 | Solute carrier family 29 member 1 |
|---|
| Type: | Multi-pass membrane protein |
|---|
| Mol. Mass.: | 50225.92 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 456 |
|---|
| Sequence: | MTTSHQPQDRYKAVWLIFFMLGLGTLLPWNFFMTATQYFTNRLDMSQNVSLVTAELSKDA
QASAAPAAPLPERNSLSAIFNNVMTLCAMLPLLLFTYLNSFLHQRIPQSVRILGSLVAIL
LVFLITAILVKVQLDALPFFVITMIKIVLINSFGAILQGSLFGLAGLLPASYTAPIMSGQ
GLAGFFASVAMICAIASGSELSESAFGYFITACAVIILTIICYLGLPRLEFYRYYQQLKL
EGPGEQETKLDLISKGEEPRAGKEESGVSVSNSQPTNESHSIKAILKNISVLAFSVCFIF
TITIGMFPAVTVEVKSSIAGSSTWERYFIPVSCFLTFNIFDWLGRSLTAVFMWPGKDSRW
LPSLVLARLVFVPLLLLCNIKPRRYLTVVFEHDAWFIFFMAAFAFSNGYLASLCMCFGPK
KVKPAEAETAGAIMAFFLCLGLALGAVFSFLFRAIV
|
|
|
|---|
| BDBM23617 |
|---|
| n/a |
|---|
| Name | BDBM23617 |
|---|
| Synonyms: | (2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-{[(4-nitrophenyl)methyl]sulfanyl}-9H-purin-9-yl)oxolane-3,4-diol | 4-nitrobenzylmercaptopurine ribonucleoside | CHEMBL418509 | NBMPR | NBTI | Nitrobenzylthioinosine |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H17N5O6S |
|---|
| Mol. Mass. | 419.412 |
|---|
| SMILES | OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(SCc3ccc(cc3)[N+]([O-])=O)ncnc12 |
|---|
| Structure | 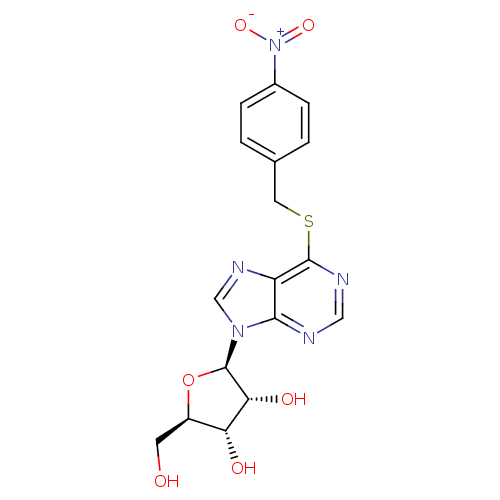
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gupte, A; Buolamwini, JK Synthesis and biological evaluation of phloridzin analogs as human concentrative nucleoside transporter 3 (hCNT3) inhibitors. Bioorg Med Chem Lett19:917-21 (2009) [PubMed] Article
Gupte, A; Buolamwini, JK Synthesis and biological evaluation of phloridzin analogs as human concentrative nucleoside transporter 3 (hCNT3) inhibitors. Bioorg Med Chem Lett19:917-21 (2009) [PubMed] Article