| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aminopeptidase N |
|---|
| Ligand | BDBM50298259 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_588447 (CHEMBL1040345) |
|---|
| IC50 | 443400±n/a nM |
|---|
| Citation |  Mou, J; Fang, H; Jing, F; Wang, Q; Liu, Y; Zhu, H; Shang, L; Wang, X; Xu, W Design, synthesis and primary activity evaluation of L-arginine derivatives as amino-peptidase N/CD13 inhibitors. Bioorg Med Chem17:4666-73 (2009) [PubMed] Article Mou, J; Fang, H; Jing, F; Wang, Q; Liu, Y; Zhu, H; Shang, L; Wang, X; Xu, W Design, synthesis and primary activity evaluation of L-arginine derivatives as amino-peptidase N/CD13 inhibitors. Bioorg Med Chem17:4666-73 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aminopeptidase N |
|---|
| Name: | Aminopeptidase N |
|---|
| Synonyms: | AMPN_PIG | ANPEP | AP-M | AP-N | Alanyl aminopeptidase | Aminopeptidase M | Aminopeptidase N (APN) | CD_antigen=CD13 | Microsomal aminopeptidase | gp130 | pAPN |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 108810.25 |
|---|
| Organism: | Sus scrofa (Pig) |
|---|
| Description: | P15145 |
|---|
| Residue: | 963 |
|---|
| Sequence: | MAKGFYISKALGILGILLGVAAVATIIALSVVYAQEKNKNAEHVPQAPTSPTITTTAAIT
LDQSKPWNRYRLPTTLLPDSYNVTLRPYLTPNADGLYIFKGKSIVRLLCQEPTDVIIIHS
KKLNYTTQGHMVVLRGVGDSQVPEIDRTELVELTEYLVVHLKGSLQPGHMYEMESEFQGE
LADDLAGFYRSEYMEGNVKKVLATTQMQSTDARKSFPCFDEPAMKATFNITLIHPNNLTA
LSNMPPKGSSTPLAEDPNWSVTEFETTPVMSTYLLAYIVSEFQSVNETAQNGVLIRIWAR
PNAIAEGHGMYALNVTGPILNFFANHYNTSYPLPKSDQIALPDFNAGAMENWGLVTYREN
ALLFDPQSSSISNKERVVTVIAHELAHQWFGNLVTLAWWNDLWLNEGFASYVEYLGADHA
EPTWNLKDLIVPGDVYRVMAVDALASSHPLTTPAEEVNTPAQISEMFDSISYSKGASVIR
MLSNFLTEDLFKEGLASYLHAFAYQNTTYLDLWEHLQKAVDAQTSIRLPDTVRAIMDRWT
LQMGFPVITVDTKTGNISQKHFLLDSESNVTRSSAFDYLWIVPISSIKNGVMQDHYWLRD
VSQAQNDLFKTASDDWVLLNVNVTGYFQVNYDEDNWRMIQHQLQTNLSVIPVINRAQVIY
DSFNLATAHMVPVTLALDNTLFLNGEKEYMPWQAALSSLSYFSLMFDRSEVYGPMKKYLR
KQVEPLFQHFETLTKNWTERPENLMDQYSEINAISTACSNGLPQCENLAKTLFDQWMSDP
ENNPIHPNLRSTIYCNAIAQGGQDQWDFAWGQLQQAQLVNEADKLRSALACSNEVWLLNR
YLGYTLNPDLIRKQDATSTINSIASNVIGQPLAWDFVQSNWKKLFQDYGGGSFSFSNLIQ
GVTRRFSSEFELQQLEQFKKNNMDVGFGSGTRALEQALEKTKANIKWVKENKEVVLNWFI
EHS
|
|
|
|---|
| BDBM50298259 |
|---|
| n/a |
|---|
| Name | BDBM50298259 |
|---|
| Synonyms: | 4-Bromo-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide | CHEMBL578858 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H17BrN6O5 |
|---|
| Mol. Mass. | 417.215 |
|---|
| SMILES | NC(NCCC[C@H](NC(=O)c1ccc(Br)cc1)C(=O)NO)=N[N+]([O-])=O |r,w:21.22| |
|---|
| Structure | 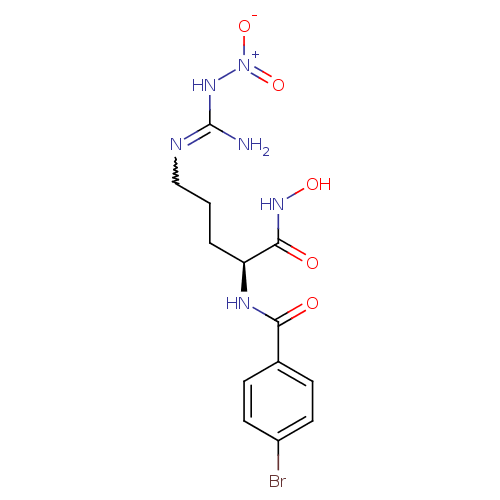
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Mou, J; Fang, H; Jing, F; Wang, Q; Liu, Y; Zhu, H; Shang, L; Wang, X; Xu, W Design, synthesis and primary activity evaluation of L-arginine derivatives as amino-peptidase N/CD13 inhibitors. Bioorg Med Chem17:4666-73 (2009) [PubMed] Article
Mou, J; Fang, H; Jing, F; Wang, Q; Liu, Y; Zhu, H; Shang, L; Wang, X; Xu, W Design, synthesis and primary activity evaluation of L-arginine derivatives as amino-peptidase N/CD13 inhibitors. Bioorg Med Chem17:4666-73 (2009) [PubMed] Article