| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50301636 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_600624 (CHEMBL1043672) |
|---|
| Ki | >1000±n/a nM |
|---|
| Citation |  Zagórska, A; Jurczyk, S; Pawlowski, M; Dybala, M; Nowak, G; Tatarczynska, E; Nikiforuk, A; Chojnacka-Wójcik, E Synthesis and preliminary pharmacological evaluation of imidazo[2,1-f]purine-2,4-dione derivatives. Eur J Med Chem44:4288-96 (2009) [PubMed] Article Zagórska, A; Jurczyk, S; Pawlowski, M; Dybala, M; Nowak, G; Tatarczynska, E; Nikiforuk, A; Chojnacka-Wójcik, E Synthesis and preliminary pharmacological evaluation of imidazo[2,1-f]purine-2,4-dione derivatives. Eur J Med Chem44:4288-96 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1 | 5-HT1A | 5-Hydroxytryptamine receptor 1A (5-HT1A) | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_RAT | 5ht1a | G-21 | Htr1a | Serotonin 1 (5-HT1) receptor | Serotonin 1a (5-HT1a) receptor/Adrenergic receptor alpha-1 | Serotonin receptor 1A |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 46445.29 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Binding assays were performed using rat hippocampal membranes. |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVFSFGQGNNTTASQEPFGTGGNVTSISDVTFSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVRKVEKKGAGT
SLGTSSAPPPKKSLNGQPGSGDWRRCAENRAVGTPCTNGAVRQGDDEATLEVIEVHRVGN
SKEHLPLPSESGSNSYAPACLERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPALLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RR
|
|
|
|---|
| BDBM50301636 |
|---|
| n/a |
|---|
| Name | BDBM50301636 |
|---|
| Synonyms: | CHEMBL566022 | N-2-[(N4-phenyl)-piperazin-N1-yl]-ethyl amideof1,3-dimethyl-6,7-dihydroimidazo[2,1-f]purine-2,4-(1H,3H)-dione-7-carboxylic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H28N8O3 |
|---|
| Mol. Mass. | 452.5095 |
|---|
| SMILES | Cn1c2nc3NC(Cn3c2c(=O)n(C)c1=O)C(=O)NCCN1CCN(CC1)c1ccccc1 |
|---|
| Structure | 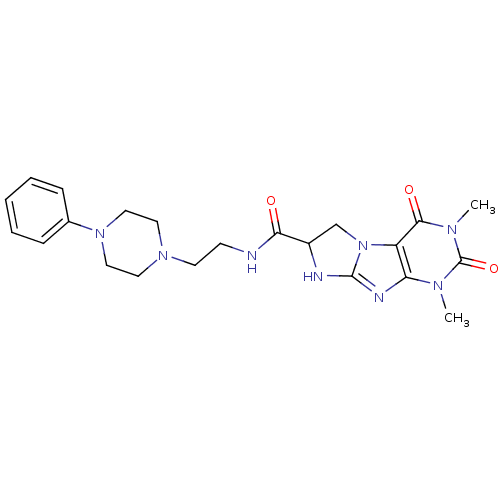
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zagórska, A; Jurczyk, S; Pawlowski, M; Dybala, M; Nowak, G; Tatarczynska, E; Nikiforuk, A; Chojnacka-Wójcik, E Synthesis and preliminary pharmacological evaluation of imidazo[2,1-f]purine-2,4-dione derivatives. Eur J Med Chem44:4288-96 (2009) [PubMed] Article
Zagórska, A; Jurczyk, S; Pawlowski, M; Dybala, M; Nowak, G; Tatarczynska, E; Nikiforuk, A; Chojnacka-Wójcik, E Synthesis and preliminary pharmacological evaluation of imidazo[2,1-f]purine-2,4-dione derivatives. Eur J Med Chem44:4288-96 (2009) [PubMed] Article