| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin G/H synthase 2 |
|---|
| Ligand | BDBM50326521 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_658294 (CHEMBL1246851) |
|---|
| IC50 | 8500±n/a nM |
|---|
| Citation |  Harrak, Y; Casula, G; Basset, J; Rosell, G; Plescia, S; Raffa, D; Cusimano, MG; Pouplana, R; Pujol, MD Synthesis, anti-inflammatory activity, and in vitro antitumor effect of a novel class of cyclooxygenase inhibitors: 4-(aryloyl)phenyl methyl sulfones. J Med Chem53:6560-71 (2010) [PubMed] Article Harrak, Y; Casula, G; Basset, J; Rosell, G; Plescia, S; Raffa, D; Cusimano, MG; Pouplana, R; Pujol, MD Synthesis, anti-inflammatory activity, and in vitro antitumor effect of a novel class of cyclooxygenase inhibitors: 4-(aryloyl)phenyl methyl sulfones. J Med Chem53:6560-71 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin G/H synthase 2 |
|---|
| Name: | Prostaglandin G/H synthase 2 |
|---|
| Synonyms: | COX2 | Cyclooxygenase-1 (COX-1) | Cyclooxygenase-2 (COX-2) | PGH2_SHEEP | PTGS2 | Prostaglandin G/H synthase (Cyclooxygenase-2) | Prostaglandin G/H synthase (cyclooxygenase) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 68976.98 |
|---|
| Organism: | Ovis aries (Sheep) |
|---|
| Description: | n/a |
|---|
| Residue: | 603 |
|---|
| Sequence: | MLARALLLCAAVVCGAANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCTTPEFLT
RIKLLLKPTPDTVHYILTHFKGVWNIVNKISFLRNMIMRYVLTSRSHLIESPPTYNVHYS
YKSWEAFSNLSYYTRALPPVPDDCPTPMGVKGRKELPDSKEVVKKVLLRRKFIPDPQGTN
LMFAFFAQHFTHQFFKTDIERGPAFTKGKNHGVDLSHVYGESLERQHNRRLFKDGKMKYQ
MINGEMYPPTVKDTQVEMIYPPHIPEHLKFAVGQEVFGLVPGLMMYATIWLREHNRVCDV
LKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQN
RIAAEFNTLYHWHPLLPDVFQIDGQEYNYQQFIYNNSVLLEHGVTQFVESFTRQIAGRVA
GRRNLPAAVEKVSKASLDQSREMKYQSFNEYRKRFLLKPYESFEELTGEKEMAAELEALY
GDIDAMELYPALLVEKPAPDAIFGETMVEAGAPFSLKGLMGNPICSPEYWKPSTFGGEVG
FKIINTASIQSLICSNVKGCPFTSFSVQDAHLTKTVTINASSSHSGLDDINPTVLLKERS
TEL
|
|
|
|---|
| BDBM50326521 |
|---|
| n/a |
|---|
| Name | BDBM50326521 |
|---|
| Synonyms: | 1-(4-(methylsulfonyl)phenyl)-1H-pyrrole | CHEMBL1240963 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C11H11NO2S |
|---|
| Mol. Mass. | 221.276 |
|---|
| SMILES | CS(=O)(=O)c1ccc(cc1)-n1cccc1 |
|---|
| Structure | 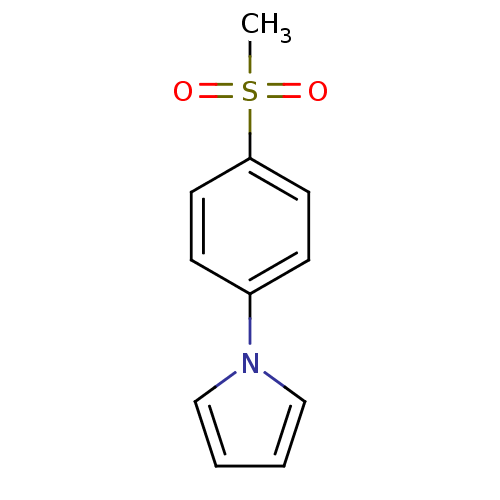
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Harrak, Y; Casula, G; Basset, J; Rosell, G; Plescia, S; Raffa, D; Cusimano, MG; Pouplana, R; Pujol, MD Synthesis, anti-inflammatory activity, and in vitro antitumor effect of a novel class of cyclooxygenase inhibitors: 4-(aryloyl)phenyl methyl sulfones. J Med Chem53:6560-71 (2010) [PubMed] Article
Harrak, Y; Casula, G; Basset, J; Rosell, G; Plescia, S; Raffa, D; Cusimano, MG; Pouplana, R; Pujol, MD Synthesis, anti-inflammatory activity, and in vitro antitumor effect of a novel class of cyclooxygenase inhibitors: 4-(aryloyl)phenyl methyl sulfones. J Med Chem53:6560-71 (2010) [PubMed] Article