| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Aryl hydrocarbon receptor |
|---|
| Ligand | BDBM50310360 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_750604 (CHEMBL1786468) |
|---|
| EC50 | 330±n/a nM |
|---|
| Citation |  Ishikawa, M; Hashimoto, Y Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J Med Chem54:1539-54 (2011) [PubMed] Article Ishikawa, M; Hashimoto, Y Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J Med Chem54:1539-54 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Aryl hydrocarbon receptor |
|---|
| Name: | Aryl hydrocarbon receptor |
|---|
| Synonyms: | AHR | AHR_HUMAN | BHLHE76 | Class E basic helix-loop-helix protein 76 | aryl hydrocarbon receptor precursor |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 96143.77 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1503828 |
|---|
| Residue: | 848 |
|---|
| Sequence: | MNSSSANITYASRKRRKPVQKTVKPIPAEGIKSNPSKRHRDRLNTELDRLASLLPFPQDV
INKLDKLSVLRLSVSYLRAKSFFDVALKSSPTERNGGQDNCRAANFREGLNLQEGEFLLQ
ALNGFVLVVTTDALVFYASSTIQDYLGFQQSDVIHQSVYELIHTEDRAEFQRQLHWALNP
SQCTESGQGIEEATGLPQTVVCYNPDQIPPENSPLMERCFICRLRCLLDNSSGFLAMNFQ
GKLKYLHGQKKKGKDGSILPPQLALFAIATPLQPPSILEIRTKNFIFRTKHKLDFTPIGC
DAKGRIVLGYTEAELCTRGSGYQFIHAADMLYCAESHIRMIKTGESGMIVFRLLTKNNRW
TWVQSNARLLYKNGRPDYIIVTQRPLTDEEGTEHLRKRNTKLPFMFTTGEAVLYEATNPF
PAIMDPLPLRTKNGTSGKDSATTSTLSKDSLNPSSLLAAMMQQDESIYLYPASSTSSTAP
FENNFFNESMNECRNWQDNTAPMGNDTILKHEQIDQPQDVNSFAGGHPGLFQDSKNSDLY
SIMKNLGIDFEDIRHMQNEKFFRNDFSGEVDFRDIDLTDEILTYVQDSLSKSPFIPSDYQ
QQQSLALNSSCMVQEHLHLEQQQQHHQKQVVVEPQQQLCQKMKHMQVNGMFENWNSNQFV
PFNCPQQDPQQYNVFTDLHGISQEFPYKSEMDSMPYTQNFISCNQPVLPQHSKCTELDYP
MGSFEPSPYPTTSSLEDFVTCLQLPENQKHGLNPQSAIITPQTCYAGAVSMYQCQPEPQH
THVGQMQYNPVLPGQQAFLNKFQNGVLNETYPAELNNINNTQTTTHLQPLHHPSEARPFP
DLTSSGFL
|
|
|
|---|
| BDBM50310360 |
|---|
| n/a |
|---|
| Name | BDBM50310360 |
|---|
| Synonyms: | 3-(2'-Fluorophenyl)-1H-naphtho[2,1-b]pyran-1-one | CHEMBL599338 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H11FO2 |
|---|
| Mol. Mass. | 290.2878 |
|---|
| SMILES | Fc1ccccc1-c1cc(=O)c2c(ccc3ccccc23)o1 |
|---|
| Structure | 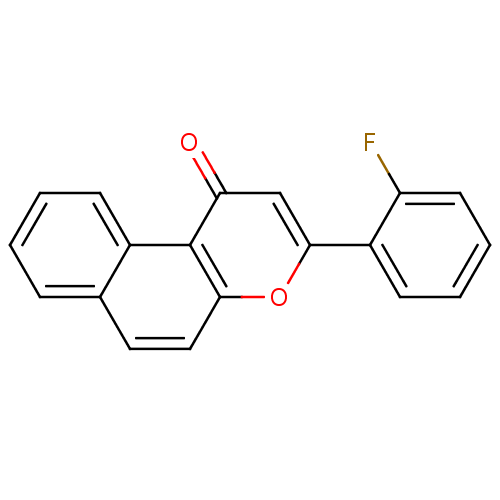
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ishikawa, M; Hashimoto, Y Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J Med Chem54:1539-54 (2011) [PubMed] Article
Ishikawa, M; Hashimoto, Y Improvement in aqueous solubility in small molecule drug discovery programs by disruption of molecular planarity and symmetry. J Med Chem54:1539-54 (2011) [PubMed] Article