| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Somatostatin receptor type 2 |
|---|
| Ligand | BDBM50352485 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_766829 (CHEMBL1827862) |
|---|
| IC50 | 35±n/a nM |
|---|
| Citation |  Erchegyi, J; Cescato, R; Waser, B; Rivier, JE; Reubi, JC N-imidazolebenzyl-histidine substitution in somatostatin and in its octapeptide analogue modulates receptor selectivity and function. J Med Chem54:5981-7 (2011) [PubMed] Article Erchegyi, J; Cescato, R; Waser, B; Rivier, JE; Reubi, JC N-imidazolebenzyl-histidine substitution in somatostatin and in its octapeptide analogue modulates receptor selectivity and function. J Med Chem54:5981-7 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Somatostatin receptor type 2 |
|---|
| Name: | Somatostatin receptor type 2 |
|---|
| Synonyms: | SOMATOSTATIN SST2 | SRIF-1 | SS-2-R | SS2-R | SS2R | SSR2_HUMAN | SSTR2 | Somatostatin receptor type 2 (SSTR2) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 41344.94 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P30874 |
|---|
| Residue: | 369 |
|---|
| Sequence: | MDMADEPLNGSHTWLSIPFDLNGSVVSTNTSNQTEPYYDLTSNAVLTFIYFVVCIIGLCG
NTLVIYVILRYAKMKTITNIYILNLAIADELFMLGLPFLAMQVALVHWPFGKAICRVVMT
VDGINQFTSIFCLTVMSIDRYLAVVHPIKSAKWRRPRTAKMITMAVWGVSLLVILPIMIY
AGLRSNQWGRSSCTINWPGESGAWYTGFIIYTFILGFLVPLTIICLCYLFIIIKVKSSGI
RVGSSKRKKSEKKVTRMVSIVVAVFIFCWLPFYIFNVSSVSMAISPTPALKGMFDFVVVL
TYANSCANPILYAFLSDNFKKSFQNVLCLVKVSGTDDGERSDSKQDKSRLNETTETQRTL
LNGDLQTSI
|
|
|
|---|
| BDBM50352485 |
|---|
| n/a |
|---|
| Name | BDBM50352485 |
|---|
| Synonyms: | CHEMBL1824049 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C74H103N17O20S2 |
|---|
| Mol. Mass. | 1614.842 |
|---|
| SMILES | C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N |r| |
|---|
| Structure | 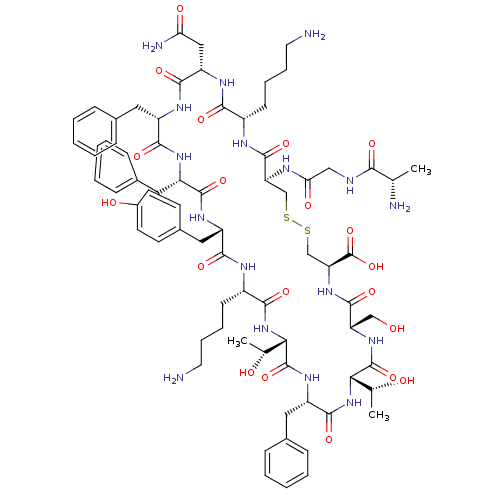
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Erchegyi, J; Cescato, R; Waser, B; Rivier, JE; Reubi, JC N-imidazolebenzyl-histidine substitution in somatostatin and in its octapeptide analogue modulates receptor selectivity and function. J Med Chem54:5981-7 (2011) [PubMed] Article
Erchegyi, J; Cescato, R; Waser, B; Rivier, JE; Reubi, JC N-imidazolebenzyl-histidine substitution in somatostatin and in its octapeptide analogue modulates receptor selectivity and function. J Med Chem54:5981-7 (2011) [PubMed] Article