| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50061220 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_50185 (CHEMBL662400) |
|---|
| IC50 | 4.7±n/a nM |
|---|
| Citation |  Bock, MG; DiPardo, RM; Evans, BE; Rittle, KE; Whitter, WL; Garsky, VM; Gilbert, KF; Leighton, JL; Carson, KL; Mellin, EC Development of 1,4-benzodiazepine cholecystokinin type B antagonists. J Med Chem36:4276-92 (1994) [PubMed] Bock, MG; DiPardo, RM; Evans, BE; Rittle, KE; Whitter, WL; Garsky, VM; Gilbert, KF; Leighton, JL; Carson, KL; Mellin, EC Development of 1,4-benzodiazepine cholecystokinin type B antagonists. J Med Chem36:4276-92 (1994) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 49676.37 |
|---|
| Organism: | RAT |
|---|
| Description: | Cholecystokinin central 0 RAT::P30551 |
|---|
| Residue: | 444 |
|---|
| Sequence: | MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQI
LLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLK
DFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAAT
WCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVM
VVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQL
SSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAE
KHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEE
DGRTIRALLSRYSYSHMSTSAPPP
|
|
|
|---|
| BDBM50061220 |
|---|
| n/a |
|---|
| Name | BDBM50061220 |
|---|
| Synonyms: | 1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-3-m-tolyl-urea | 1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-3-m-tolyl-urea | 1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-3-m-tolyl-urea((S)L365_260) | CHEMBL70380 | L-365,260 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H22N4O2 |
|---|
| Mol. Mass. | 398.4571 |
|---|
| SMILES | CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| |
|---|
| Structure | 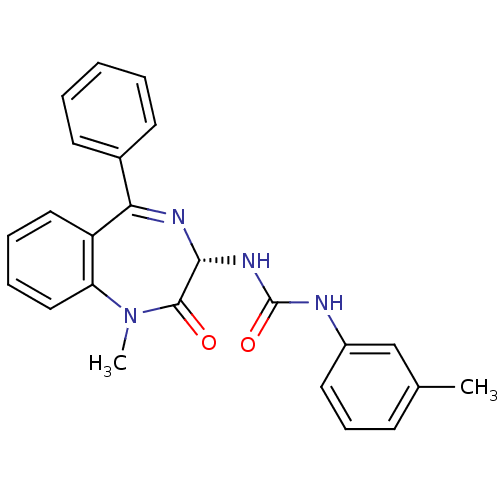
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bock, MG; DiPardo, RM; Evans, BE; Rittle, KE; Whitter, WL; Garsky, VM; Gilbert, KF; Leighton, JL; Carson, KL; Mellin, EC Development of 1,4-benzodiazepine cholecystokinin type B antagonists. J Med Chem36:4276-92 (1994) [PubMed]
Bock, MG; DiPardo, RM; Evans, BE; Rittle, KE; Whitter, WL; Garsky, VM; Gilbert, KF; Leighton, JL; Carson, KL; Mellin, EC Development of 1,4-benzodiazepine cholecystokinin type B antagonists. J Med Chem36:4276-92 (1994) [PubMed]