| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50179023 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_29993 (CHEMBL642181) |
|---|
| Ki | 1793±n/a nM |
|---|
| Citation |  Baraldi, PG; Cacciari, B; Pineda de Las Infantas, MJ; Romagnoli, R; Spalluto, G; Volpini, R; Costanzi, S; Vittori, S; Cristalli, G; Melman, N; Park, KS; Ji, XD; Jacobson, KA Synthesis and biological activity of a new series of N6-arylcarbamoyl, 2-(Ar)alkynyl-N6-arylcarbamoyl, and N6-carboxamido derivatives of adenosine-5'-N-ethyluronamide as A1 and A3 adenosine receptor agonists. J Med Chem41:3174-85 (1998) [PubMed] Article Baraldi, PG; Cacciari, B; Pineda de Las Infantas, MJ; Romagnoli, R; Spalluto, G; Volpini, R; Costanzi, S; Vittori, S; Cristalli, G; Melman, N; Park, KS; Ji, XD; Jacobson, KA Synthesis and biological activity of a new series of N6-arylcarbamoyl, 2-(Ar)alkynyl-N6-arylcarbamoyl, and N6-carboxamido derivatives of adenosine-5'-N-ethyluronamide as A1 and A3 adenosine receptor agonists. J Med Chem41:3174-85 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | AA2AR_RAT | ADENOSINE A2a | Adenosine A2 receptor | Adenosine A2a receptor (A2a) | Adenosine Receptors A2a (A2a) | Adenosine receptor A2a and A3 | Adenosine receptors A2a | Adora2a | Rat striatal adenosine A2a receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 45015.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat A2A receptors expressed in CHO cells. |
|---|
| Residue: | 410 |
|---|
| Sequence: | MGSSVYITVELAIAVLAILGNVLVCWAVWINSNLQNVTNFFVVSLAAADIAVGVLAIPFA
ITISTGFCAACHGCLFFACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGVRAKG
IIAICWVLSFAIGLTPMLGWNNCSQKDGNSTKTCGEGRVTCLFEDVVPMNYMVYYNFFAF
VLLPLLLMLAIYLRIFLAARRQLKQMESQPLPGERTRSTLQKEVHAAKSLAIIVGLFALC
WLPLHIINCFTFFCSTCRHAPPWLMYLAIILSHSNSVVNPFIYAYRIREFRQTFRKIIRT
HVLRRQEPFQAGGSSAWALAAHSTEGEQVSLRLNGHPLGVWANGSATHSGRRPNGYTLGL
GGGGSAQGSPRDVELPTQERQEGQEHPGLRGHLVQARVGASSWSSEFAPS
|
|
|
|---|
| BDBM50179023 |
|---|
| n/a |
|---|
| Name | BDBM50179023 |
|---|
| Synonyms: | 1-benzyl-3-(9-((2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-tetrahydrofuran-2-yl)-9H-purin-6-yl)urea | CHEMBL426847 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H23N7O5 |
|---|
| Mol. Mass. | 441.4405 |
|---|
| SMILES | CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC(=O)NCc3ccccc3)ncnc12 |
|---|
| Structure | 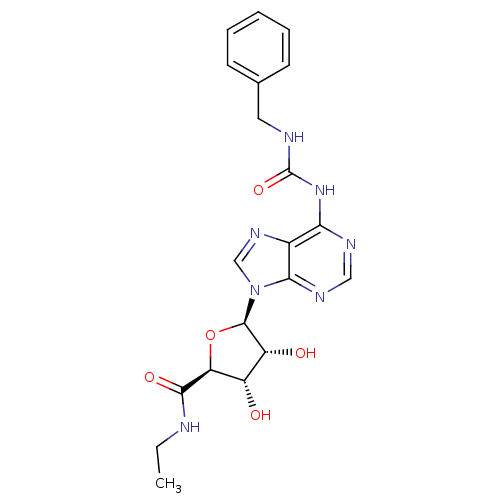
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Baraldi, PG; Cacciari, B; Pineda de Las Infantas, MJ; Romagnoli, R; Spalluto, G; Volpini, R; Costanzi, S; Vittori, S; Cristalli, G; Melman, N; Park, KS; Ji, XD; Jacobson, KA Synthesis and biological activity of a new series of N6-arylcarbamoyl, 2-(Ar)alkynyl-N6-arylcarbamoyl, and N6-carboxamido derivatives of adenosine-5'-N-ethyluronamide as A1 and A3 adenosine receptor agonists. J Med Chem41:3174-85 (1998) [PubMed] Article
Baraldi, PG; Cacciari, B; Pineda de Las Infantas, MJ; Romagnoli, R; Spalluto, G; Volpini, R; Costanzi, S; Vittori, S; Cristalli, G; Melman, N; Park, KS; Ji, XD; Jacobson, KA Synthesis and biological activity of a new series of N6-arylcarbamoyl, 2-(Ar)alkynyl-N6-arylcarbamoyl, and N6-carboxamido derivatives of adenosine-5'-N-ethyluronamide as A1 and A3 adenosine receptor agonists. J Med Chem41:3174-85 (1998) [PubMed] Article