| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glucocorticoid receptor |
|---|
| Ligand | BDBM50150339 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_306508 (CHEMBL828113) |
|---|
| IC50 | 220±n/a nM |
|---|
| Citation |  Link, JT; Sorensen, BK; Lai, C; Wang, J; Fung, S; Deng, D; Emery, M; Carroll, S; Grynfarb, M; Goos-Nilsson, A; Von Geldern, T Synthesis, activity, metabolic stability, and pharmacokinetics of glucocorticoid receptor modulator-statin hybrids. Bioorg Med Chem Lett14:4173-8 (2004) [PubMed] Article Link, JT; Sorensen, BK; Lai, C; Wang, J; Fung, S; Deng, D; Emery, M; Carroll, S; Grynfarb, M; Goos-Nilsson, A; Von Geldern, T Synthesis, activity, metabolic stability, and pharmacokinetics of glucocorticoid receptor modulator-statin hybrids. Bioorg Med Chem Lett14:4173-8 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Glucocorticoid receptor |
|---|
| Name: | Glucocorticoid receptor |
|---|
| Synonyms: | GCR_RAT | Glucocorticoid | Glucocorticoid Receptor (GR) | Glucocorticoid receptor | Grl | Nr3c1 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 87556.83 |
|---|
| Organism: | RAT |
|---|
| Description: | Glucocorticoid 0 RAT::P06536 |
|---|
| Residue: | 795 |
|---|
| Sequence: | MDSKESLAPPGRDEVPGSLLGQGRGSVMDFYKSLRGGATVKVSASSPSVAAASQADSKQQ
RILLDFSKGSTSNVQQRQQQQQQQQQQQQQQQQQQQPDLSKAVSLSMGLYMGETETKVMG
NDLGYPQQGQLGLSSGETDFRLLEESIANLNRSTSVPENPKSSTSATGCATPTEKEFPKT
HSDASSEQQNRKSQTGTNGGSVKLYPTDQSTFDLLKDLEFSAGSPSKDTNESPWRSDLLI
DENLLSPLAGEDDPFLLEGNTNEDCKPLILPDTKPKIKDTGDTILSSPSSVALPQVKTEK
DDFIELCTPGVIKQEKLGPVYCQASFSGTNIIGNKMSAISVHGVSTSGGQMYHYDMNTAS
LSQQQDQKPVFNVIPPIPVGSENWNRCQGSGEDSLTSLGALNFPGRSVFSNGYSSPGMRP
DVSSPPSSSSAATGPPPKLCLVCSDEASGCHYGVLTCGSCKVFFKRAVEGQHNYLCAGRN
DCIIDKIRRKNCPACRYRKCLQAGMNLEARKTKKKIKGIQQATAGVSQDTSENPNKTIVP
AALPQLTPTLVSLLEVIEPEVLYAGYDSSVPDSAWRIMTTLNMLGGRQVIAAVKWAKAIL
GLRNLHLDDQMTLLQYSWMFLMAFALGWRSYRQSSGNLLCFAPDLIINEQRMSLPCMYDQ
CKHMLFVSSELQRLQVSYEEYLCMKTLLLLSSVPKEGLKSQELFDEIRMTYIKELGKAIV
KREGNSSQNWQRFYQLTKLLDSMHEVVENLLTYCFQTFLDKTMSIEFPEMLAEIITNQIP
KYSNGNIKKLLFHQK
|
|
|
|---|
| BDBM50150339 |
|---|
| n/a |
|---|
| Name | BDBM50150339 |
|---|
| Synonyms: | (S)-3-Hydroxy-4-{[4-((8S,11R,13S,14S,17S)-17-hydroxy-13-methyl-3-oxo-17-prop-1-ynyl-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-11-yl)-phenyl]-methyl-amino}-butyric acid | CHEMBL361406 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C32H39NO5 |
|---|
| Mol. Mass. | 517.6558 |
|---|
| SMILES | CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)C[C@@H](O)CC(O)=O |c:18,t:11| |
|---|
| Structure | 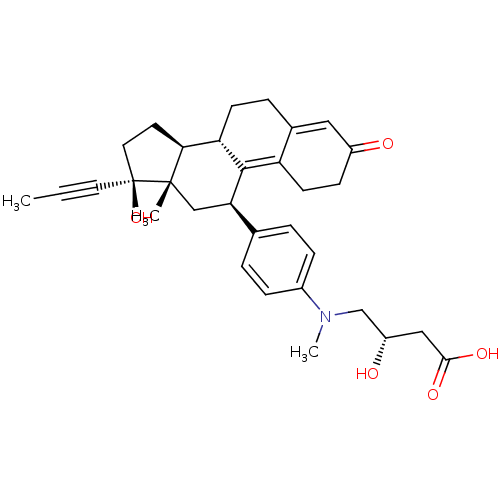
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Link, JT; Sorensen, BK; Lai, C; Wang, J; Fung, S; Deng, D; Emery, M; Carroll, S; Grynfarb, M; Goos-Nilsson, A; Von Geldern, T Synthesis, activity, metabolic stability, and pharmacokinetics of glucocorticoid receptor modulator-statin hybrids. Bioorg Med Chem Lett14:4173-8 (2004) [PubMed] Article
Link, JT; Sorensen, BK; Lai, C; Wang, J; Fung, S; Deng, D; Emery, M; Carroll, S; Grynfarb, M; Goos-Nilsson, A; Von Geldern, T Synthesis, activity, metabolic stability, and pharmacokinetics of glucocorticoid receptor modulator-statin hybrids. Bioorg Med Chem Lett14:4173-8 (2004) [PubMed] Article