| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nitric oxide synthase, endothelial |
|---|
| Ligand | BDBM50066778 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_305165 (CHEMBL832755) |
|---|
| IC50 | 520±n/a nM |
|---|
| Citation |  Shankaran, K; Donnelly, KL; Shah, SK; Guthikonda, RN; MacCoss, M; Humes, JL; Pacholok, SG; Grant, SK; Kelly, TM; Wong, KK Evaluation of pyrrolidin-2-imines and 1,3-thiazolidin-2-imines as inhibitors of nitric oxide synthase. Bioorg Med Chem Lett14:4539-44 (2004) [PubMed] Article Shankaran, K; Donnelly, KL; Shah, SK; Guthikonda, RN; MacCoss, M; Humes, JL; Pacholok, SG; Grant, SK; Kelly, TM; Wong, KK Evaluation of pyrrolidin-2-imines and 1,3-thiazolidin-2-imines as inhibitors of nitric oxide synthase. Bioorg Med Chem Lett14:4539-44 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nitric oxide synthase, endothelial |
|---|
| Name: | Nitric oxide synthase, endothelial |
|---|
| Synonyms: | Constitutive NOS | EC-NOS | Endothelial NOS | Endothelial nitric oxide synthase | NOS type III | NOS3 | NOS3_HUMAN | NOSIII | Nitric oxide synthase (inducible and endothelial) | Nitric oxide synthase, endothelial (eNOS) | Nitric-oxide synthase (endothelial and brain) | cNOS | eNOS |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 133297.84 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29474 |
|---|
| Residue: | 1203 |
|---|
| Sequence: | MGNLKSVAQEPGPPCGLGLGLGLGLCGKQGPATPAPEPSRAPASLLPPAPEHSPPSSPLT
QPPEGPKFPRVKNWEVGSITYDTLSAQAQQDGPCTPRRCLGSLVFPRKLQGRPSPGPPAP
EQLLSQARDFINQYYSSIKRSGSQAHEQRLQEVEAEVAATGTYQLRESELVFGAKQAWRN
APRCVGRIQWGKLQVFDARDCRSAQEMFTYICNHIKYATNRGNLRSAITVFPQRCPGRGD
FRIWNSQLVRYAGYRQQDGSVRGDPANVEITELCIQHGWTPGNGRFDVLPLLLQAPDDPP
ELFLLPPELVLEVPLEHPTLEWFAALGLRWYALPAVSNMLLEIGGLEFPAAPFSGWYMST
EIGTRNLCDPHRYNILEDVAVCMDLDTRTTSSLWKDKAAVEINVAVLHSYQLAKVTIVDH
HAATASFMKHLENEQKARGGCPADWAWIVPPISGSLTPVFHQEMVNYFLSPAFRYQPDPW
KGSAAKGTGITRKKTFKEVANAVKISASLMGTVMAKRVKATILYGSETGRAQSYAQQLGR
LFRKAFDPRVLCMDEYDVVSLEHETLVLVVTSTFGNGDPPENGESFAAALMEMSGPYNSS
PRPEQHKSYKIRFNSISCSDPLVSSWRRKRKESSNTDSAGALGTLRFCVFGLGSRAYPHF
CAFARAVDTRLEELGGERLLQLGQGDELCGQEEAFRGWAQAAFQAACETFCVGEDAKAAA
RDIFSPKRSWKRQRYRLSAQAEGLQLLPGLIHVHRRKMFQATIRSVENLQSSKSTRATIL
VRLDTGGQEGLQYQPGDHIGVCPPNRPGLVEALLSRVEDPPAPTEPVAVEQLEKGSPGGP
PPGWVRDPRLPPCTLRQALTFFLDITSPPSPQLLRLLSTLAEEPREQQELEALSQDPRRY
EEWKWFRCPTLLEVLEQFPSVALPAPLLLTQLPLLQPRYYSVSSAPSTHPGEIHLTVAVL
AYRTQDGLGPLHYGVCSTWLSQLKPGDPVPCFIRGAPSFRLPPDPSLPCILVGPGTGIAP
FRGFWQERLHDIESKGLQPTPMTLVFGCRCSQLDHLYRDEVQNAQQRGVFGRVLTAFSRE
PDNPKTYVQDILRTELAAEVHRVLCLERGHMFVCGDVTMATNVLQTVQRILATEGDMELD
EAGDVIGVLRDQQRYHEDIFGLTLRTQEVTSRIRTQSFSLQERQLRGAVPWAFDPPGSDT
NSP
|
|
|
|---|
| BDBM50066778 |
|---|
| n/a |
|---|
| Name | BDBM50066778 |
|---|
| Synonyms: | 5-Ethyl-4-methyl-pyrrolidin-(2E)-ylideneamine; hydrochloride | CHEMBL185024 | CHEMBL552935 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C7H14N2 |
|---|
| Mol. Mass. | 126.1995 |
|---|
| SMILES | CCC1N=C(N)CC1C |t:3| |
|---|
| Structure | 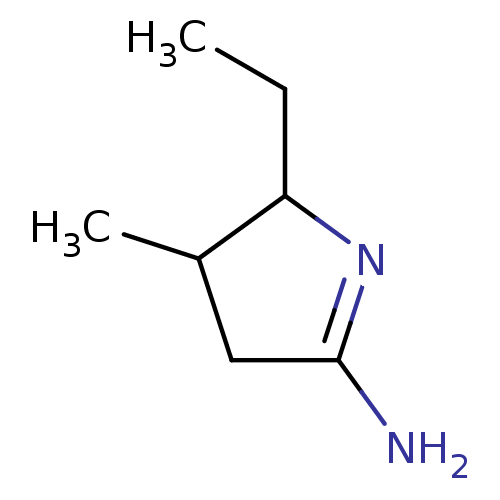
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Shankaran, K; Donnelly, KL; Shah, SK; Guthikonda, RN; MacCoss, M; Humes, JL; Pacholok, SG; Grant, SK; Kelly, TM; Wong, KK Evaluation of pyrrolidin-2-imines and 1,3-thiazolidin-2-imines as inhibitors of nitric oxide synthase. Bioorg Med Chem Lett14:4539-44 (2004) [PubMed] Article
Shankaran, K; Donnelly, KL; Shah, SK; Guthikonda, RN; MacCoss, M; Humes, JL; Pacholok, SG; Grant, SK; Kelly, TM; Wong, KK Evaluation of pyrrolidin-2-imines and 1,3-thiazolidin-2-imines as inhibitors of nitric oxide synthase. Bioorg Med Chem Lett14:4539-44 (2004) [PubMed] Article