| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-galactosidase |
|---|
| Ligand | BDBM50118923 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_305098 |
|---|
| IC50 | 18000000000±n/a nM |
|---|
| Citation |  Ogawa, S; Funayama, S; Okazaki, K; Ishizuka, F; Sakata, Y; Doi, F Synthesis of 5a-carba-hexopyranoses and hexopyranosylamines, as well as 5a,5a'-dicarbadisaccharides, from 3,8-dioxatricyclo[4.2.1.0(2,4)]nonan-9-ol: glycosidase inhibitory activity of N-substituted 5a-carba-beta-gluco- and beta-galactopyranosylamines, and derivatives thereof. Bioorg Med Chem Lett14:5183-8 (2004) [PubMed] Article Ogawa, S; Funayama, S; Okazaki, K; Ishizuka, F; Sakata, Y; Doi, F Synthesis of 5a-carba-hexopyranoses and hexopyranosylamines, as well as 5a,5a'-dicarbadisaccharides, from 3,8-dioxatricyclo[4.2.1.0(2,4)]nonan-9-ol: glycosidase inhibitory activity of N-substituted 5a-carba-beta-gluco- and beta-galactopyranosylamines, and derivatives thereof. Bioorg Med Chem Lett14:5183-8 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Beta-galactosidase |
|---|
| Name: | Beta-galactosidase |
|---|
| Synonyms: | β-galactosidase | BGAL_BOVIN | Beta-galactosidase/Glucosylceramidase | GLB1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 73420.88 |
|---|
| Organism: | Bos taurus (Bovine) |
|---|
| Description: | n/a |
|---|
| Residue: | 653 |
|---|
| Sequence: | MPGVVRLLALLLVPLLLGSARGLHNATQRTFQIDYRRNRFLKDGQPFRYISGSIHYFRVP
RFYWKDRLLKMKMAGLNAIQTYVAWNFHELQPGRYNFSGDHDVEHFIQLAHELGLLVILR
PGPYICAEWDMGGLPAWLLEKKSIVLRSSDPDYLAAVDKWLGVLLPKMRPLLYKNGGPII
TVQVENEYGSYLSCDYDYLRFLQKRFHDHLGEDVLLFTTDGVNERLLQCGALQGLYATVD
FSPGTNLTAAFMLQRKFEPTGPLVNSEFYTGWLDHWGQRHSTVSSKAVAFTLHDMLALGA
NVNMYMFIGGTNFAYWNGANIPYQPQPTSYDYDAPLSEAGDLTEKYFALRDIIQKFAKVP
EGPIPPSTPKFAYGKVALNKLKTVEDALNILCPSGPIKSVYPLTFIDVKQYFGFVLYRTM
LPEDCSDPTPLSSPLSGVHDRAYVSVNGVAQGILERESVITLNITGKAGATLDLLVENMG
RVNYGSSINDFKGLVSNLTLGSKILTNWEIFPLDMEDAVRSHLGTWGGRDRGYHNKARAH
SPPTYALPTFYVGNFTIPSGIADLPQDTFIQFPGWTKGQVWINGFNLGRYWPVRGPQMTL
FVPQHILVTSTPNTIVVLELEHAPCQDGGPELCTVEFVDKPVFRTVQTHRHAN
|
|
|
|---|
| BDBM50118923 |
|---|
| n/a |
|---|
| Name | BDBM50118923 |
|---|
| Synonyms: | (1S,2S,3R)-4-Methyl-6-octylamino-cyclohexane-1,2,3-triol | 4-Methyl-6-octylamino-cyclohexane-1,2,3-triol | CHEMBL314757 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H31NO3 |
|---|
| Mol. Mass. | 273.4115 |
|---|
| SMILES | CCCCCCCCNC1CC(C)C(O)C(O)C1O |
|---|
| Structure | 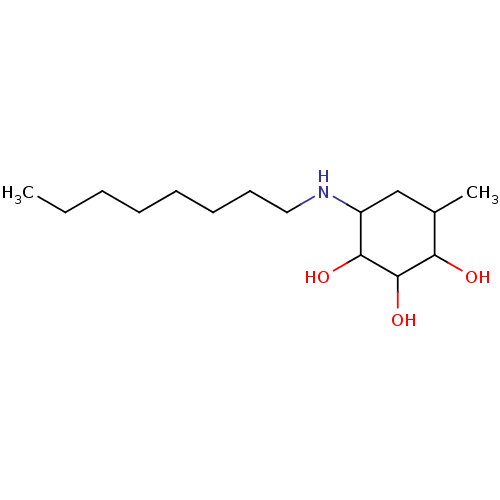
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ogawa, S; Funayama, S; Okazaki, K; Ishizuka, F; Sakata, Y; Doi, F Synthesis of 5a-carba-hexopyranoses and hexopyranosylamines, as well as 5a,5a'-dicarbadisaccharides, from 3,8-dioxatricyclo[4.2.1.0(2,4)]nonan-9-ol: glycosidase inhibitory activity of N-substituted 5a-carba-beta-gluco- and beta-galactopyranosylamines, and derivatives thereof. Bioorg Med Chem Lett14:5183-8 (2004) [PubMed] Article
Ogawa, S; Funayama, S; Okazaki, K; Ishizuka, F; Sakata, Y; Doi, F Synthesis of 5a-carba-hexopyranoses and hexopyranosylamines, as well as 5a,5a'-dicarbadisaccharides, from 3,8-dioxatricyclo[4.2.1.0(2,4)]nonan-9-ol: glycosidase inhibitory activity of N-substituted 5a-carba-beta-gluco- and beta-galactopyranosylamines, and derivatives thereof. Bioorg Med Chem Lett14:5183-8 (2004) [PubMed] Article