

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Nitric oxide synthase, endothelial | ||
| Ligand | BDBM50355312 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_772642 (CHEMBL1839728) | ||
| IC50 | 46200±n/a nM | ||
| Citation |  Annedi, SC; Maddaford, SP; Mladenova, G; Ramnauth, J; Rakhit, S; Andrews, JS; Lee, DK; Zhang, D; Porreca, F; Bunton, D; Christie, L Discovery of N-(3-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indol-6-yl) thiophene-2-carboximidamide as a selective inhibitor of human neuronal nitric oxide synthase (nNOS) for the treatment of pain. J Med Chem54:7408-16 (2011) [PubMed] Article Annedi, SC; Maddaford, SP; Mladenova, G; Ramnauth, J; Rakhit, S; Andrews, JS; Lee, DK; Zhang, D; Porreca, F; Bunton, D; Christie, L Discovery of N-(3-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indol-6-yl) thiophene-2-carboximidamide as a selective inhibitor of human neuronal nitric oxide synthase (nNOS) for the treatment of pain. J Med Chem54:7408-16 (2011) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Nitric oxide synthase, endothelial | |||
| Name: | Nitric oxide synthase, endothelial | ||
| Synonyms: | Constitutive NOS | EC-NOS | Endothelial NOS | Endothelial nitric oxide synthase | NOS type III | NOS3 | NOS3_HUMAN | NOSIII | Nitric oxide synthase (inducible and endothelial) | Nitric oxide synthase, endothelial (eNOS) | Nitric-oxide synthase (endothelial and brain) | cNOS | eNOS | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 133297.84 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P29474 | ||
| Residue: | 1203 | ||
| Sequence: |
| ||
| BDBM50355312 | |||
| n/a | |||
| Name | BDBM50355312 | ||
| Synonyms: | CHEMBL1835115 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H20N4S | ||
| Mol. Mass. | 348.465 | ||
| SMILES | NC(=Nc1ccc2c(c[nH]c2c1)C1=CN2CCC1CC2)c1cccs1 |w:2.2,t:14,TLB:7:12:16.15:18.19,(3.98,-16.46,;3.98,-18,;5.31,-18.77,;6.65,-18.01,;6.64,-16.48,;7.97,-15.7,;9.31,-16.48,;10.78,-16.01,;11.68,-17.26,;10.76,-18.5,;9.3,-18.02,;7.98,-18.78,;11.27,-14.55,;11.08,-13.17,;12.55,-12.53,;13.89,-13.13,;14.18,-14.53,;12.81,-13.9,;13.06,-12,;12.62,-10.89,;2.64,-18.77,;2.16,-20.23,;.61,-20.23,;.14,-18.76,;1.39,-17.85,)| | ||
| Structure | 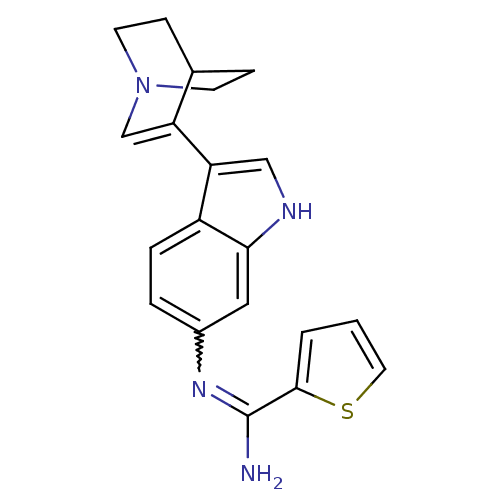
| ||