| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase Lck |
|---|
| Ligand | BDBM50335179 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_815113 (CHEMBL2024838) |
|---|
| IC50 | 12±n/a nM |
|---|
| Citation |  Voss, ME; Rainka, MP; Fleming, M; Peterson, LH; Belanger, DB; Siddiqui, MA; Hruza, A; Voigt, J; Gray, K; Basso, AD Synthesis and SAR studies of imidazo-[1,2-a]-pyrazine Aurora kinase inhibitors with improved off-target kinase selectivity. Bioorg Med Chem Lett22:3544-9 (2012) [PubMed] Article Voss, ME; Rainka, MP; Fleming, M; Peterson, LH; Belanger, DB; Siddiqui, MA; Hruza, A; Voigt, J; Gray, K; Basso, AD Synthesis and SAR studies of imidazo-[1,2-a]-pyrazine Aurora kinase inhibitors with improved off-target kinase selectivity. Bioorg Med Chem Lett22:3544-9 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tyrosine-protein kinase Lck |
|---|
| Name: | Tyrosine-protein kinase Lck |
|---|
| Synonyms: | 2.7.10.2 | LCK | LCK_HUMAN | LSK | Leukocyte C-terminal Src kinase | Lymphocyte cell-specific protein-tyrosine kinase | Lymphocyte-specific protein tyrosine kinase | P56-LCK | Protein YT16 | Proto-oncogene Lck | Proto-oncogene tyrosine-protein kinase LCK | Src/Lck kinase | T cell-specific protein-tyrosine kinase |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 57987.83 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P06239 |
|---|
| Residue: | 509 |
|---|
| Sequence: | MGCGCSSHPEDDWMENIDVCENCHYPIVPLDGKGTLLIRNGSEVRDPLVTYEGSNPPASP
LQDNLVIALHSYEPSHDGDLGFEKGEQLRILEQSGEWWKAQSLTTGQEGFIPFNFVAKAN
SLEPEPWFFKNLSRKDAERQLLAPGNTHGSFLIRESESTAGSFSLSVRDFDQNQGEVVKH
YKIRNLDNGGFYISPRITFPGLHELVRHYTNASDGLCTRLSRPCQTQKPQKPWWEDEWEV
PRETLKLVERLGAGQFGEVWMGYYNGHTKVAVKSLKQGSMSPDAFLAEANLMKQLQHQRL
VRLYAVVTQEPIYIITEYMENGSLVDFLKTPSGIKLTINKLLDMAAQIAEGMAFIEERNY
IHRDLRAANILVSDTLSCKIADFGLARLIEDNEYTAREGAKFPIKWTAPEAINYGTFTIK
SDVWSFGILLTEIVTHGRIPYPGMTNPEVIQNLERGYRMVRPDNCPEELYQLMRLCWKER
PEDRPTFDYLRSVLEDFFTATEGQYQPQP
|
|
|
|---|
| BDBM50335179 |
|---|
| n/a |
|---|
| Name | BDBM50335179 |
|---|
| Synonyms: | 6-Methyl-N-[3-(1-piperidinylmethyl)-5-isothiazolyl]-3-(1Hpyrazol-4-yl)imidazo[1,2-a]pyrazin-8-amine Hydrochloride | CHEMBL1650536 | CHEMBL1739550 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H22N8S |
|---|
| Mol. Mass. | 394.497 |
|---|
| SMILES | Cc1cn2c(cnc2c(Nc2cc(CN3CCCCC3)ns2)n1)-c1cn[nH]c1 |
|---|
| Structure | 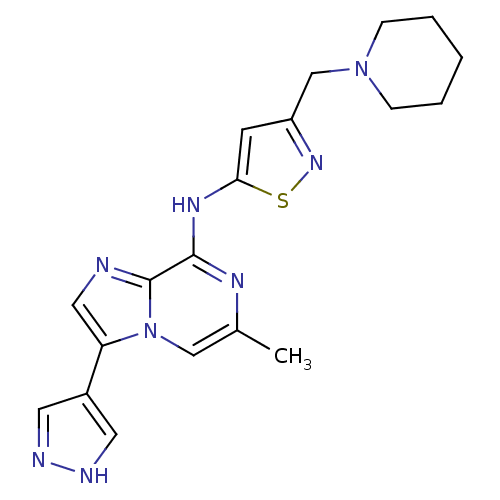
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Voss, ME; Rainka, MP; Fleming, M; Peterson, LH; Belanger, DB; Siddiqui, MA; Hruza, A; Voigt, J; Gray, K; Basso, AD Synthesis and SAR studies of imidazo-[1,2-a]-pyrazine Aurora kinase inhibitors with improved off-target kinase selectivity. Bioorg Med Chem Lett22:3544-9 (2012) [PubMed] Article
Voss, ME; Rainka, MP; Fleming, M; Peterson, LH; Belanger, DB; Siddiqui, MA; Hruza, A; Voigt, J; Gray, K; Basso, AD Synthesis and SAR studies of imidazo-[1,2-a]-pyrazine Aurora kinase inhibitors with improved off-target kinase selectivity. Bioorg Med Chem Lett22:3544-9 (2012) [PubMed] Article