| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phosphatidylinositol 5-phosphate 4-kinase type-2 gamma |
|---|
| Ligand | BDBM50401152 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_881256 (CHEMBL2211988) |
|---|
| IC50 | 1072±n/a nM |
|---|
| Citation |  Sunose, M; Bell, K; Ellard, K; Bergamini, G; Neubauer, G; Werner, T; Ramsden, N Discovery of 5-(2-amino-[1,2,4]triazolo[1,5-a]pyridin-7-yl)-N-(tert-butyl)pyridine-3-sulfonamide (CZC24758), as a potent, orally bioavailable and selective inhibitor of PI3K for the treatment of inflammatory disease. Bioorg Med Chem Lett22:4613-8 (2012) [PubMed] Article Sunose, M; Bell, K; Ellard, K; Bergamini, G; Neubauer, G; Werner, T; Ramsden, N Discovery of 5-(2-amino-[1,2,4]triazolo[1,5-a]pyridin-7-yl)-N-(tert-butyl)pyridine-3-sulfonamide (CZC24758), as a potent, orally bioavailable and selective inhibitor of PI3K for the treatment of inflammatory disease. Bioorg Med Chem Lett22:4613-8 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phosphatidylinositol 5-phosphate 4-kinase type-2 gamma |
|---|
| Name: | Phosphatidylinositol 5-phosphate 4-kinase type-2 gamma |
|---|
| Synonyms: | PI(5)P 4-kinase type II gamma | PI42C_HUMAN | PIP4K2C | PIP4KII-gamma | PIP5K2C | Phosphatidylinositol-5-phosphate 4-kinase type II gamma | Phosphatidylinositol-5-phosphate 4-kinase type-2 gamma |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 47302.35 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_774614 |
|---|
| Residue: | 421 |
|---|
| Sequence: | MASSSVPPATVSAATAGPGPGFGFASKTKKKHFVQQKVKVFRAADPLVGVFLWGVAHSIN
ELSQVPPPVMLLPDDFKASSKIKVNNHLFHRENLPSHFKFKEYCPQVFRNLRDRFGIDDQ
DYLVSLTRNPPSESEGSDGRFLISYDRTLVIKEVSSEDIADMHSNLSNYHQYIVKCHGNT
LLPQFLGMYRVSVDNEDSYMLVMRNMFSHRLPVHRKYDLKGSLVSREASDKEKVKELPTL
KDMDFLNKNQKVYIGEEEKKIFLEKLKRDVEFLVQLKIMDYSLLLGIHDIIRGSEPEEEA
PVREDESEVDGDCSLTGPPALVGSYGTSPEGIGGYIHSHRPLGPGEFESFIDVYAIRSAE
GAPQKEVYFMGLIDILTQYDAKKKAAHAAKTVKHGAGAEISTVHPEQYAKRFLDFITNIF
A
|
|
|
|---|
| BDBM50401152 |
|---|
| n/a |
|---|
| Name | BDBM50401152 |
|---|
| Synonyms: | CHEMBL2205766 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H18N6O2S |
|---|
| Mol. Mass. | 346.407 |
|---|
| SMILES | CC(C)(C)NS(=O)(=O)c1cncc(c1)-c1ccn2nc(N)nc2c1 |
|---|
| Structure | 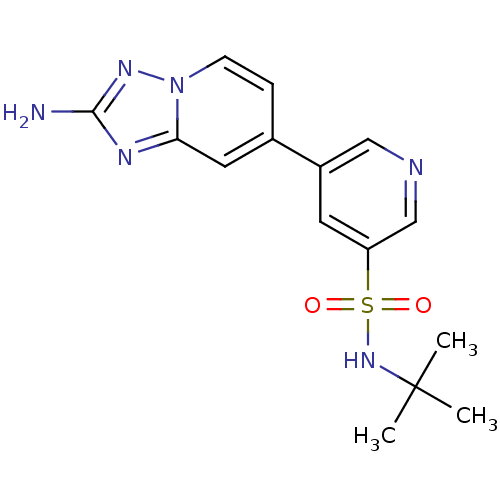
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sunose, M; Bell, K; Ellard, K; Bergamini, G; Neubauer, G; Werner, T; Ramsden, N Discovery of 5-(2-amino-[1,2,4]triazolo[1,5-a]pyridin-7-yl)-N-(tert-butyl)pyridine-3-sulfonamide (CZC24758), as a potent, orally bioavailable and selective inhibitor of PI3K for the treatment of inflammatory disease. Bioorg Med Chem Lett22:4613-8 (2012) [PubMed] Article
Sunose, M; Bell, K; Ellard, K; Bergamini, G; Neubauer, G; Werner, T; Ramsden, N Discovery of 5-(2-amino-[1,2,4]triazolo[1,5-a]pyridin-7-yl)-N-(tert-butyl)pyridine-3-sulfonamide (CZC24758), as a potent, orally bioavailable and selective inhibitor of PI3K for the treatment of inflammatory disease. Bioorg Med Chem Lett22:4613-8 (2012) [PubMed] Article