| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2B |
|---|
| Ligand | BDBM50416679 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_651970 (CHEMBL1227643) |
|---|
| Ki | 25.12±n/a nM |
|---|
| Citation |  Gianotti, M; Corti, C; Delle Fratte, S; Di Fabio, R; Leslie, CP; Pavone, F; Piccoli, L; Stasi, L; Wigglesworth, MJ Novel imidazobenzazepine derivatives as dual H1/5-HT2A antagonists for the treatment of sleep disorders. Bioorg Med Chem Lett20:5069-73 (2010) [PubMed] Article Gianotti, M; Corti, C; Delle Fratte, S; Di Fabio, R; Leslie, CP; Pavone, F; Piccoli, L; Stasi, L; Wigglesworth, MJ Novel imidazobenzazepine derivatives as dual H1/5-HT2A antagonists for the treatment of sleep disorders. Bioorg Med Chem Lett20:5069-73 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2B |
|---|
| Name: | 5-hydroxytryptamine receptor 2B |
|---|
| Synonyms: | 5-HT-2B | 5-HT2B | 5-hydroxytryptamine (serotonin) receptor 2B [Homo sapiens] | 5-hydroxytryptamine receptor 2B (5-HT2B) | 5-hydroxytryptamine receptor 2C (5HT2C) | 5HT2B_HUMAN | HTR2B | Serotonin (5-HT3) receptor | Serotonin 2b (5-HT2b) receptor | Serotonin Receptor 2B |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 54312.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Receptor binding assays were performed using human clone stably expressed in CHO cells. |
|---|
| Residue: | 481 |
|---|
| Sequence: | MALSYRVSELQSTIPEHILQSTFVHVISSNWSGLQTESIPEEMKQIVEEQGNKLHWAALL
ILMVIIPTIGGNTLVILAVSLEKKLQYATNYFLMSLAVADLLVGLFVMPIALLTIMFEAM
WPLPLVLCPAWLFLDVLFSTASIMHLCAISVDRYIAIKKPIQANQYNSRATAFIKITVVW
LISIGIAIPVPIKGIETDVDNPNNITCVLTKERFGDFMLFGSLAAFFTPLAIMIVTYFLT
IHALQKKAYLVKNKPPQRLTWLTVSTVFQRDETPCSSPEKVAMLDGSRKDKALPNSGDET
LMRRTSTIGKKSVQTISNEQRASKVLGIVFFLFLLMWCPFFITNITLVLCDSCNQTTLQM
LLEIFVWIGYVSSGVNPLVYTLFNKTFRDAFGRYITCNYRATKSVKTLRKRSSKIYFRNP
MAENSKFFKKHGIRNGINPAMYQSPMRLRSSTIQSSSIILLDTLLLTENEGDKTEEQVSY
V
|
|
|
|---|
| BDBM50416679 |
|---|
| n/a |
|---|
| Name | BDBM50416679 |
|---|
| Synonyms: | CHEMBL1222762 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H25ClN4O2 |
|---|
| Mol. Mass. | 400.902 |
|---|
| SMILES | CC(C)(CN1CCN(CC1)C1=Cc2ccccc2Cn2c(Cl)cnc12)C(O)=O |t:11| |
|---|
| Structure | 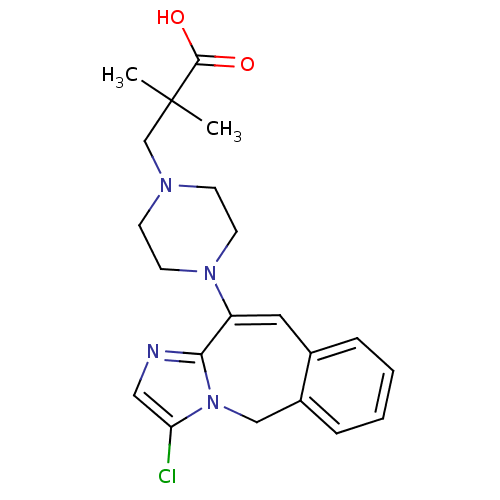
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gianotti, M; Corti, C; Delle Fratte, S; Di Fabio, R; Leslie, CP; Pavone, F; Piccoli, L; Stasi, L; Wigglesworth, MJ Novel imidazobenzazepine derivatives as dual H1/5-HT2A antagonists for the treatment of sleep disorders. Bioorg Med Chem Lett20:5069-73 (2010) [PubMed] Article
Gianotti, M; Corti, C; Delle Fratte, S; Di Fabio, R; Leslie, CP; Pavone, F; Piccoli, L; Stasi, L; Wigglesworth, MJ Novel imidazobenzazepine derivatives as dual H1/5-HT2A antagonists for the treatment of sleep disorders. Bioorg Med Chem Lett20:5069-73 (2010) [PubMed] Article