| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM50272612 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1348900 (CHEMBL3271169) |
|---|
| IC50 | 29400±n/a nM |
|---|
| Citation |  Jabeen, F; Oliferenko, PV; Oliferenko, AA; Pillai, GG; Ansari, FL; Hall, CD; Katritzky, AR Dual inhibition of thea-glucosidase and butyrylcholinesterase studied by molecular field topology analysis. Eur J Med Chem80:228-42 (2014) [PubMed] Article Jabeen, F; Oliferenko, PV; Oliferenko, AA; Pillai, GG; Ansari, FL; Hall, CD; Katritzky, AR Dual inhibition of thea-glucosidase and butyrylcholinesterase studied by molecular field topology analysis. Eur J Med Chem80:228-42 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | Acylcholine acylhydrolase | BCHE | Butyrylcholine esterase (BChE) | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | CHE1 | CHLE_HUMAN | Choline esterase II | Cholinesterases | Cholinesterases; ACHE & BCHE | Pseudocholinesterase |
|---|
| Type: | Homotetramer |
|---|
| Mol. Mass.: | 68422.27 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P06276 |
|---|
| Residue: | 602 |
|---|
| Sequence: | MHSKVTIICIRFLFWFLLLCMLIGKSHTEDDIIIATKNGKVRGMNLTVFGGTVTAFLGIP
YAQPPLGRLRFKKPQSLTKWSDIWNATKYANSCCQNIDQSFPGFHGSEMWNPNTDLSEDC
LYLNVWIPAPKPKNATVLIWIYGGGFQTGTSSLHVYDGKFLARVERVIVVSMNYRVGALG
FLALPGNPEAPGNMGLFDQQLALQWVQKNIAAFGGNPKSVTLFGESAGAASVSLHLLSPG
SHSLFTRAILQSGSFNAPWAVTSLYEARNRTLNLAKLTGCSRENETEIIKCLRNKDPQEI
LLNEAFVVPYGTPLSVNFGPTVDGDFLTDMPDILLELGQFKKTQILVGVNKDEGTAFLVY
GAPGFSKDNNSIITRKEFQEGLKIFFPGVSEFGKESILFHYTDWVDDQRPENYREALGDV
VGDYNFICPALEFTKKFSEWGNNAFFYYFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLER
RDNYTKAEEILSRSIVKRWANFAKYGNPNETQNNSTSWPVFKSTEQKYLTLNTESTRIMT
KLRAQQCRFWTSFFPKVLEMTGNIDEAEWEWKAGFHRWNNYMMDWKNQFNDYTSKKESCV
GL
|
|
|
|---|
| BDBM50272612 |
|---|
| n/a |
|---|
| Name | BDBM50272612 |
|---|
| Synonyms: | 2-(3''-Chlorophenyl)-4-(3'-hydroxyphenyl)-2,3-dihydro-1,5-benzothiazepine | CHEMBL525905 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H16ClNOS |
|---|
| Mol. Mass. | 365.876 |
|---|
| SMILES | Oc1cccc(c1)C1=Nc2ccccc2SC(C1)c1cccc(Cl)c1 |t:8| |
|---|
| Structure | 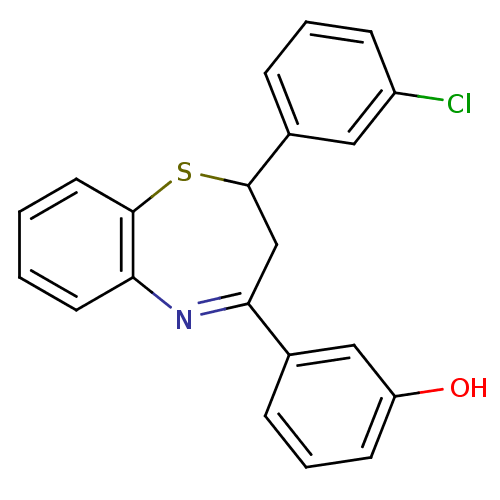
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jabeen, F; Oliferenko, PV; Oliferenko, AA; Pillai, GG; Ansari, FL; Hall, CD; Katritzky, AR Dual inhibition of thea-glucosidase and butyrylcholinesterase studied by molecular field topology analysis. Eur J Med Chem80:228-42 (2014) [PubMed] Article
Jabeen, F; Oliferenko, PV; Oliferenko, AA; Pillai, GG; Ansari, FL; Hall, CD; Katritzky, AR Dual inhibition of thea-glucosidase and butyrylcholinesterase studied by molecular field topology analysis. Eur J Med Chem80:228-42 (2014) [PubMed] Article