

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Melatonin receptor type 1A | ||
| Ligand | BDBM50139845 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEBML_1553992 | ||
| Ki | 120±n/a nM | ||
| Citation |  Couhert, A; Delagrange, P; Caignard, DH; Chartier, A; Suzenet, F; Guillaumet, G Synthesis of 2-arylfuro[3,2-b]pyridines: Effect of the C2-aryl group on melatoninergic activity. Eur J Med Chem109:268-75 (2016) [PubMed] Article Couhert, A; Delagrange, P; Caignard, DH; Chartier, A; Suzenet, F; Guillaumet, G Synthesis of 2-arylfuro[3,2-b]pyridines: Effect of the C2-aryl group on melatoninergic activity. Eur J Med Chem109:268-75 (2016) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Melatonin receptor type 1A | |||
| Name: | Melatonin receptor type 1A | ||
| Synonyms: | MTNR1A | MTNR1A protein | MTR1A_HUMAN | Mel-1A-R | Mel1a melatonin receptor | Melatonin 1A | Melatonin receptor | Melatonin receptor 1A | Melatonin receptor type 1 (MT1) | Melatonin receptor type 1A | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 39392.94 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P48039 | ||
| Residue: | 350 | ||
| Sequence: |
| ||
| BDBM50139845 | |||
| n/a | |||
| Name | BDBM50139845 | ||
| Synonyms: | CHEMBL3764421 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C20H22N2O3 | ||
| Mol. Mass. | 338.4003 | ||
| SMILES | COc1ccc2oc(c(CCNC(C)=O)c2n1)-c1c(C)cccc1C |(-3.72,-2.76,;-3.72,-1.53,;-2.38,-.77,;-2.38,.77,;-1.03,1.55,;.3,.77,;1.76,1.24,;2.66,.02,;1.76,-1.24,;2.24,-2.7,;3.75,-3.01,;4.24,-4.47,;5.75,-4.78,;6.57,-3.86,;6.13,-5.95,;.3,-.77,;-1.03,-1.55,;4.2,.03,;4.87,-1.36,;4.18,-2.38,;6.41,-1.46,;7.27,-.19,;6.59,1.2,;5.05,1.3,;4.51,2.41,)| | ||
| Structure | 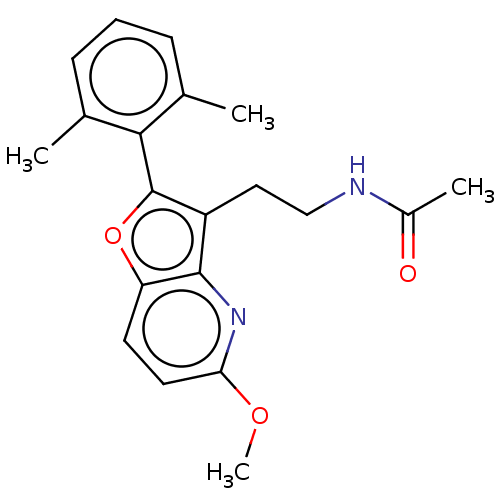
| ||