| Synonyms: | PI3-kinase p110 subunit gamma | PI3-kinase subunit p120-gamma | PI3Kgamma | PIK3CG | PK3CG_HUMAN | Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase 110 kDa catalytic subunit gamma (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma (PI3Kgamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3Kgamma) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Phosphoinositide 3-Kinase (PI3K), gamma Chain A | Phosphoinositide 3-kinases gamma (PI3K gamma) | Phosphoinositide-3-kinase (PI3K gamma) | p120-PI3K |
|---|
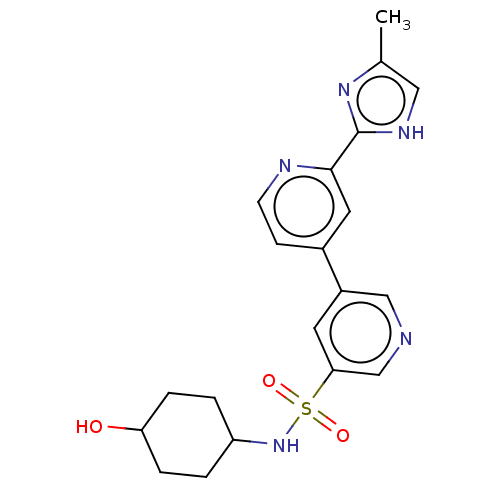
| SMILES | Cc1c[nH]c(n1)-c1cc(ccn1)-c1cncc(c1)S(=O)(=O)NC1CCC(O)CC1 |(9.34,4.93,;9.34,3.39,;8.09,2.48,;8.57,1.02,;10.11,1.02,;10.58,2.48,;11.01,-.23,;10.38,-1.63,;11.29,-2.88,;12.82,-2.72,;13.45,-1.31,;12.54,-.06,;10.66,-4.29,;11.57,-5.53,;10.94,-6.94,;9.41,-7.1,;8.51,-5.85,;9.13,-4.45,;6.97,-6.01,;6.81,-4.48,;7.13,-7.55,;5.44,-6.18,;4.54,-4.93,;3.01,-5.09,;2.1,-3.84,;2.73,-2.44,;1.82,-1.19,;4.26,-2.28,;5.16,-3.52,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sparks, RB; Shepard, S; Combs, AP; Buesking, AW; Shao, L; Wang, H; Falahatpisheh, N Pyridine and pyridimine compounds as PI3K-gamma inhibitors US Patent US11352340 Publication Date 6/7/2022
Sparks, RB; Shepard, S; Combs, AP; Buesking, AW; Shao, L; Wang, H; Falahatpisheh, N Pyridine and pyridimine compounds as PI3K-gamma inhibitors US Patent US11352340 Publication Date 6/7/2022