| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Proteasome subunit beta type-5 |
|---|
| Ligand | BDBM651113 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | IC50 Determination |
|---|
| IC50 | 140±n/a nM |
|---|
| Citation |  LIN, G; NATHAN, C; KIRKMAN, L; ZHAN, W; MORGAN, T; SATO, K; HARA, R; KAWASAKI, M; IMAEDA, T; TOITA, A; OKAMOTO, R; YUKAWA, T; ASO, K; WONG, T; GINN, JD; FOLEY, MA MACROCYCLIC COMPOUNDS AS PROTEASOME INHIBITORS US Patent US20240043470 Publication Date 2/8/2024 LIN, G; NATHAN, C; KIRKMAN, L; ZHAN, W; MORGAN, T; SATO, K; HARA, R; KAWASAKI, M; IMAEDA, T; TOITA, A; OKAMOTO, R; YUKAWA, T; ASO, K; WONG, T; GINN, JD; FOLEY, MA MACROCYCLIC COMPOUNDS AS PROTEASOME INHIBITORS US Patent US20240043470 Publication Date 2/8/2024 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Proteasome subunit beta type-5 |
|---|
| Name: | Proteasome subunit beta type-5 |
|---|
| Synonyms: | 20S proteasome chymotrypsin-like | 26S proteosome | LMPX | MB1 | PSB5_HUMAN | PSMB5 | Proteasome Macropain subunit MB1 | Proteasome subunit beta type-1/beta type-5 | X |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 28480.96 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 263 |
|---|
| Sequence: | MALASVLERPLPVNQRGFFGLGGRADLLDLGPGSLSDGLSLAAPGWGVPEEPGIEMLHGT
TTLAFKFRHGVIVAADSRATAGAYIASQTVKKVIEINPYLLGTMAGGAADCSFWERLLAR
QCRIYELRNKERISVAAASKLLANMVYQYKGMGLSMGTMICGWDKRGPGLYYVDSEGNRI
SGATFSVGSGSVYAYGVMDRGYSYDLEVEQAYDLARRAIYQATYRDAYSGGAVNLYHVRE
DGWIRVSSDNVADLHEKYSGSTP
|
|
|
|---|
| BDBM651113 |
|---|
| n/a |
|---|
| Name | BDBM651113 |
|---|
| Synonyms: | US20240043470, Compound 1-66 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H38F5N5O5 |
|---|
| Mol. Mass. | 679.6773 |
|---|
| SMILES | FC(F)(F)CNC(=O)[C@@H]1Cc2cccc(Oc3cccc(C[C@H](N4CCCC4=O)C(=O)N[C@@H](CCN4CCC(F)(F)CC4)C(=O)N1)c3)c2 |r| |
|---|
| Structure | 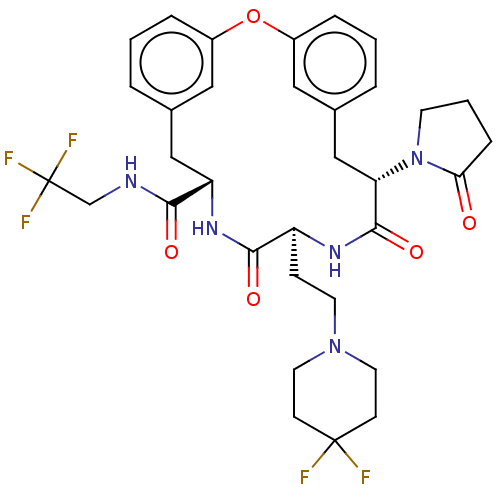
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 LIN, G; NATHAN, C; KIRKMAN, L; ZHAN, W; MORGAN, T; SATO, K; HARA, R; KAWASAKI, M; IMAEDA, T; TOITA, A; OKAMOTO, R; YUKAWA, T; ASO, K; WONG, T; GINN, JD; FOLEY, MA MACROCYCLIC COMPOUNDS AS PROTEASOME INHIBITORS US Patent US20240043470 Publication Date 2/8/2024
LIN, G; NATHAN, C; KIRKMAN, L; ZHAN, W; MORGAN, T; SATO, K; HARA, R; KAWASAKI, M; IMAEDA, T; TOITA, A; OKAMOTO, R; YUKAWA, T; ASO, K; WONG, T; GINN, JD; FOLEY, MA MACROCYCLIC COMPOUNDS AS PROTEASOME INHIBITORS US Patent US20240043470 Publication Date 2/8/2024