| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor gamma |
|---|
| Ligand | BDBM50103636 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1793746 (CHEMBL4265665) |
|---|
| IC50 | >5000±n/a nM |
|---|
| Citation |  Tanis, SP; Colca, JR; Parker, TT; Artman, GD; Larsen, SD; McDonald, WG; Gadwood, RC; Kletzien, RF; Zeller, JB; Lee, PH; Adams, WJ PPAR?-sparing thiazolidinediones as insulin sensitizers. Design, synthesis and selection of compounds for clinical development. Bioorg Med Chem26:5870-5884 (2018) [PubMed] Article Tanis, SP; Colca, JR; Parker, TT; Artman, GD; Larsen, SD; McDonald, WG; Gadwood, RC; Kletzien, RF; Zeller, JB; Lee, PH; Adams, WJ PPAR?-sparing thiazolidinediones as insulin sensitizers. Design, synthesis and selection of compounds for clinical development. Bioorg Med Chem26:5870-5884 (2018) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor gamma |
|---|
| Name: | Peroxisome proliferator-activated receptor gamma |
|---|
| Synonyms: | NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARγ) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2 |
|---|
| Type: | Nuclear Receptor |
|---|
| Mol. Mass.: | 57613.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P37231 |
|---|
| Residue: | 505 |
|---|
| Sequence: | MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSF
DIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKT
QLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNC
RIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLR
ALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQE
QSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLAS
LMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVII
LSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQL
LQVIKKTETDMSLHPLLQEIYKDLY
|
|
|
|---|
| BDBM50103636 |
|---|
| n/a |
|---|
| Name | BDBM50103636 |
|---|
| Synonyms: | ADD-3878 | CHEBI:64227 | Ciglitazone | U-63287 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H23NO3S |
|---|
| Mol. Mass. | 333.445 |
|---|
| SMILES | CC1(COc2ccc(CC3SC(=O)NC3=O)cc2)CCCCC1 |
|---|
| Structure | 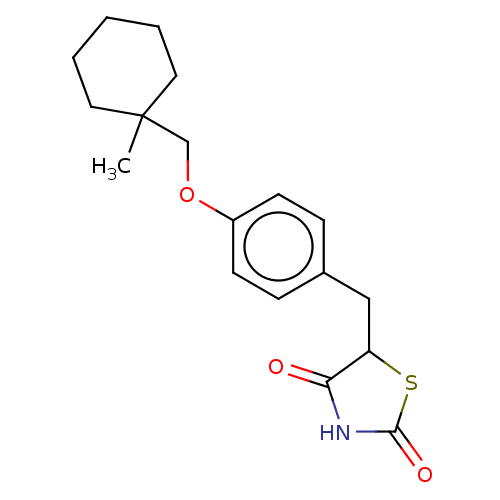
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tanis, SP; Colca, JR; Parker, TT; Artman, GD; Larsen, SD; McDonald, WG; Gadwood, RC; Kletzien, RF; Zeller, JB; Lee, PH; Adams, WJ PPAR?-sparing thiazolidinediones as insulin sensitizers. Design, synthesis and selection of compounds for clinical development. Bioorg Med Chem26:5870-5884 (2018) [PubMed] Article
Tanis, SP; Colca, JR; Parker, TT; Artman, GD; Larsen, SD; McDonald, WG; Gadwood, RC; Kletzien, RF; Zeller, JB; Lee, PH; Adams, WJ PPAR?-sparing thiazolidinediones as insulin sensitizers. Design, synthesis and selection of compounds for clinical development. Bioorg Med Chem26:5870-5884 (2018) [PubMed] Article