| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2B |
|---|
| Ligand | BDBM50497430 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1440700 (CHEMBL3381335) |
|---|
| Ki | 0.510000±n/a nM |
|---|
| Citation |  Moritomo, A; Yamada, H; Matsuzawa-Nomura, T; Watanabe, T; Itahana, H; Oku, M; Akuzawa, S; Okada, M Synthesis and pharmacological evaluation of optically pure, novel carbonyl guanidine derivatives as dual 5-HT2B and 5-HT7 receptor antagonists. Bioorg Med Chem22:6026-38 (2014) [PubMed] Article Moritomo, A; Yamada, H; Matsuzawa-Nomura, T; Watanabe, T; Itahana, H; Oku, M; Akuzawa, S; Okada, M Synthesis and pharmacological evaluation of optically pure, novel carbonyl guanidine derivatives as dual 5-HT2B and 5-HT7 receptor antagonists. Bioorg Med Chem22:6026-38 (2014) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2B |
|---|
| Name: | 5-hydroxytryptamine receptor 2B |
|---|
| Synonyms: | 5-HT-2B | 5-HT2B | 5-hydroxytryptamine (serotonin) receptor 2B [Homo sapiens] | 5-hydroxytryptamine receptor 2B (5-HT2B) | 5-hydroxytryptamine receptor 2C (5HT2C) | 5HT2B_HUMAN | HTR2B | Serotonin (5-HT3) receptor | Serotonin 2b (5-HT2b) receptor | Serotonin Receptor 2B |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 54312.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Receptor binding assays were performed using human clone stably expressed in CHO cells. |
|---|
| Residue: | 481 |
|---|
| Sequence: | MALSYRVSELQSTIPEHILQSTFVHVISSNWSGLQTESIPEEMKQIVEEQGNKLHWAALL
ILMVIIPTIGGNTLVILAVSLEKKLQYATNYFLMSLAVADLLVGLFVMPIALLTIMFEAM
WPLPLVLCPAWLFLDVLFSTASIMHLCAISVDRYIAIKKPIQANQYNSRATAFIKITVVW
LISIGIAIPVPIKGIETDVDNPNNITCVLTKERFGDFMLFGSLAAFFTPLAIMIVTYFLT
IHALQKKAYLVKNKPPQRLTWLTVSTVFQRDETPCSSPEKVAMLDGSRKDKALPNSGDET
LMRRTSTIGKKSVQTISNEQRASKVLGIVFFLFLLMWCPFFITNITLVLCDSCNQTTLQM
LLEIFVWIGYVSSGVNPLVYTLFNKTFRDAFGRYITCNYRATKSVKTLRKRSSKIYFRNP
MAENSKFFKKHGIRNGINPAMYQSPMRLRSSTIQSSSIILLDTLLLTENEGDKTEEQVSY
V
|
|
|
|---|
| BDBM50497430 |
|---|
| n/a |
|---|
| Name | BDBM50497430 |
|---|
| Synonyms: | CHEMBL3343668 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H16ClN3O |
|---|
| Mol. Mass. | 313.781 |
|---|
| SMILES | Cl.[#7]\[#6](-[#7])=[#7]\[#6](=O)-c1ccc2-c3ccccc3C3([#6]-[#6]3)c2c1 |
|---|
| Structure | 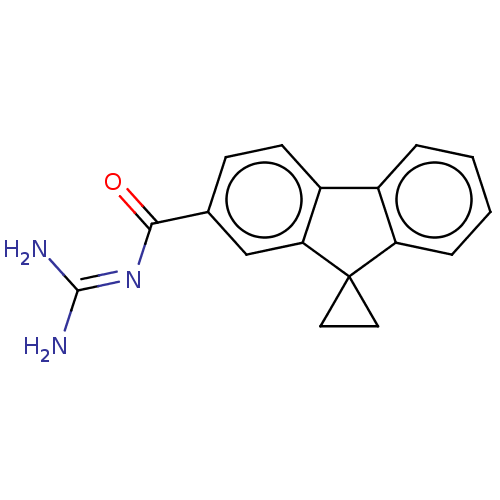
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Moritomo, A; Yamada, H; Matsuzawa-Nomura, T; Watanabe, T; Itahana, H; Oku, M; Akuzawa, S; Okada, M Synthesis and pharmacological evaluation of optically pure, novel carbonyl guanidine derivatives as dual 5-HT2B and 5-HT7 receptor antagonists. Bioorg Med Chem22:6026-38 (2014) [PubMed] Article
Moritomo, A; Yamada, H; Matsuzawa-Nomura, T; Watanabe, T; Itahana, H; Oku, M; Akuzawa, S; Okada, M Synthesis and pharmacological evaluation of optically pure, novel carbonyl guanidine derivatives as dual 5-HT2B and 5-HT7 receptor antagonists. Bioorg Med Chem22:6026-38 (2014) [PubMed] Article