| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 1A2 |
|---|
| Ligand | BDBM50526314 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1899806 (CHEMBL4401921) |
|---|
| IC50 | >25000±n/a nM |
|---|
| Citation |  Goncalves, MB; Clarke, E; Jarvis, CI; Barret Kalindjian, S; Pitcher, T; Grist, J; Hobbs, C; Carlstedt, T; Jack, J; Brown, JT; Mills, M; Mumford, P; Borthwick, AD; Corcoran, JPT Discovery and lead optimisation of a potent, selective and orally bioavailable RAR? agonist for the potential treatment of nerve injury. Bioorg Med Chem Lett29:995-1000 (2019) [PubMed] Article Goncalves, MB; Clarke, E; Jarvis, CI; Barret Kalindjian, S; Pitcher, T; Grist, J; Hobbs, C; Carlstedt, T; Jack, J; Brown, JT; Mills, M; Mumford, P; Borthwick, AD; Corcoran, JPT Discovery and lead optimisation of a potent, selective and orally bioavailable RAR? agonist for the potential treatment of nerve injury. Bioorg Med Chem Lett29:995-1000 (2019) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 1A2 |
|---|
| Name: | Cytochrome P450 1A2 |
|---|
| Synonyms: | CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 58423.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P05177 |
|---|
| Residue: | 516 |
|---|
| Sequence: | MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKN
PHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDG
QSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELM
AGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFP
ILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGN
LIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLS
DRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPEL
WEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLE
FSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
|
|
|
|---|
| BDBM50526314 |
|---|
| n/a |
|---|
| Name | BDBM50526314 |
|---|
| Synonyms: | CHEMBL4459692 | US10752616, Code No. BHBA-001 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H14N2O4 |
|---|
| Mol. Mass. | 334.3255 |
|---|
| SMILES | Cc1ccc(C)c2oc(cc12)-c1nc(no1)-c1ccc(cc1)C(O)=O |
|---|
| Structure | 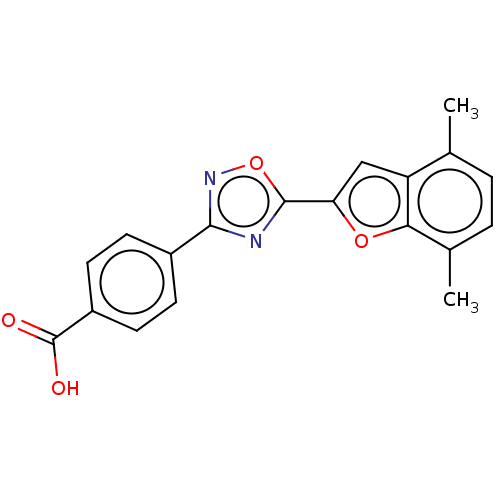
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Goncalves, MB; Clarke, E; Jarvis, CI; Barret Kalindjian, S; Pitcher, T; Grist, J; Hobbs, C; Carlstedt, T; Jack, J; Brown, JT; Mills, M; Mumford, P; Borthwick, AD; Corcoran, JPT Discovery and lead optimisation of a potent, selective and orally bioavailable RAR? agonist for the potential treatment of nerve injury. Bioorg Med Chem Lett29:995-1000 (2019) [PubMed] Article
Goncalves, MB; Clarke, E; Jarvis, CI; Barret Kalindjian, S; Pitcher, T; Grist, J; Hobbs, C; Carlstedt, T; Jack, J; Brown, JT; Mills, M; Mumford, P; Borthwick, AD; Corcoran, JPT Discovery and lead optimisation of a potent, selective and orally bioavailable RAR? agonist for the potential treatment of nerve injury. Bioorg Med Chem Lett29:995-1000 (2019) [PubMed] Article