| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2A |
|---|
| Ligand | BDBM50115645 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2679 (CHEMBL617927) |
|---|
| Ki | 277±n/a nM |
|---|
| Citation |  Wikström, HV; Mensonides-Harsema, MM; Cremers, TI; Moltzen, EK; Arnt, J Synthesis and pharmacological testing of 1,2,3,4,10,14b-hexahydro-6-methoxy-2-methyldibenzo[c,f]pyrazino[1,2-a]azepin and its enantiomers in comparison with the two antidepressants mianserin and mirtazapine. J Med Chem45:3280-5 (2002) [PubMed] Wikström, HV; Mensonides-Harsema, MM; Cremers, TI; Moltzen, EK; Arnt, J Synthesis and pharmacological testing of 1,2,3,4,10,14b-hexahydro-6-methoxy-2-methyldibenzo[c,f]pyrazino[1,2-a]azepin and its enantiomers in comparison with the two antidepressants mianserin and mirtazapine. J Med Chem45:3280-5 (2002) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2A |
|---|
| Name: | 5-hydroxytryptamine receptor 2A |
|---|
| Synonyms: | 5-HT-2A | 5-HT2 | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_RAT | Htr2 | Htr2a | Serotonin Receptor 2A |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 52852.05 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat cortex membranes 5-HT2A receptors. |
|---|
| Residue: | 471 |
|---|
| Sequence: | MEILCEDNISLSSIPNSLMQLGDGPRLYHNDFNSRDANTSEASNWTIDAENRTNLSCEGY
LPPTCLSILHLQEKNWSALLTTVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIAD
MLLGFLVMPVSMLTILYGYRWPLPSKLCAIWIYLDVLFSTASIMHLCAISLDRYVAIQNP
IHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSF
VAFFIPLTIMVITYFLTIKSLQKEATLCVSDLSTRAKLASFSFLPQSSLSSEKLFQRSIH
REPGSYAGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNENVIGA
LLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENRKPLQLILVNTIPALAYK
SSQLQVGQKKNSQEDAEQTVDDCSMVTLGKQQSEENCTDNIETVNEKVSCV
|
|
|
|---|
| BDBM50115645 |
|---|
| n/a |
|---|
| Name | BDBM50115645 |
|---|
| Synonyms: | (+/-)-8Trifluoro-methanesulfonic acid 2-methyl-1,2,3,4,9,13b-hexahydro-2,4a-diaza-tribenzo[a,c,e]cyclohepten-5-yl ester | CHEMBL109877 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H19F3N2O3S |
|---|
| Mol. Mass. | 412.426 |
|---|
| SMILES | CN1CCN2C(C1)c1ccccc1Cc1cccc(OS(=O)(=O)C(F)(F)F)c21 |
|---|
| Structure | 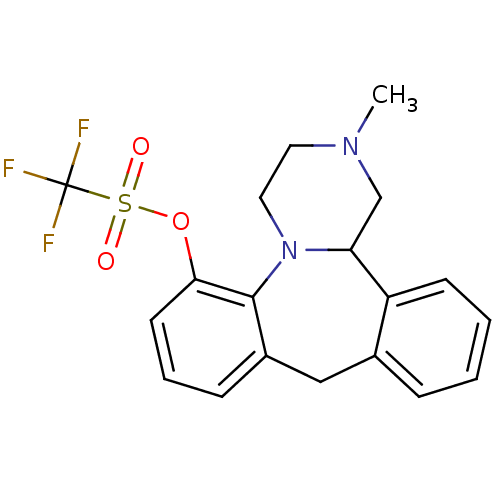
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wikström, HV; Mensonides-Harsema, MM; Cremers, TI; Moltzen, EK; Arnt, J Synthesis and pharmacological testing of 1,2,3,4,10,14b-hexahydro-6-methoxy-2-methyldibenzo[c,f]pyrazino[1,2-a]azepin and its enantiomers in comparison with the two antidepressants mianserin and mirtazapine. J Med Chem45:3280-5 (2002) [PubMed]
Wikström, HV; Mensonides-Harsema, MM; Cremers, TI; Moltzen, EK; Arnt, J Synthesis and pharmacological testing of 1,2,3,4,10,14b-hexahydro-6-methoxy-2-methyldibenzo[c,f]pyrazino[1,2-a]azepin and its enantiomers in comparison with the two antidepressants mianserin and mirtazapine. J Med Chem45:3280-5 (2002) [PubMed]