| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prothrombin |
|---|
| Ligand | BDBM50322033 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1510507 (CHEMBL3607635) |
|---|
| IC50 | 5300±n/a nM |
|---|
| Citation |  Aoyama, H; Ijuin, R; Kato, JY; Urushiyama, S; Tetsuhashi, M; Hashimoto, Y; Yokomatsu, T Discovery of non-competitive thrombin inhibitor derived from competitive tryptase inhibitor skeleton: Shift in molecular recognition resulted from skeletal conversion of carboxylate into phosphonate. Bioorg Med Chem Lett25:3676-80 (2015) [PubMed] Article Aoyama, H; Ijuin, R; Kato, JY; Urushiyama, S; Tetsuhashi, M; Hashimoto, Y; Yokomatsu, T Discovery of non-competitive thrombin inhibitor derived from competitive tryptase inhibitor skeleton: Shift in molecular recognition resulted from skeletal conversion of carboxylate into phosphonate. Bioorg Med Chem Lett25:3676-80 (2015) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prothrombin |
|---|
| Name: | Prothrombin |
|---|
| Synonyms: | Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 70029.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00734 |
|---|
| Residue: | 622 |
|---|
| Sequence: | MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLEREC
VEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHV
NITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQE
CSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASA
QAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETG
DGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYI
DGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTEN
DLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHP
VCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDST
RIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKY
GFYTHVFRLKKWIQKVIDQFGE
|
|
|
|---|
| BDBM50322033 |
|---|
| n/a |
|---|
| Name | BDBM50322033 |
|---|
| Synonyms: | 2-Methylisoindole-1,3-dione-5-yl 3-(6-aminopyridin-3-yl)propionate | CHEMBL1171655 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H15N3O4 |
|---|
| Mol. Mass. | 325.3187 |
|---|
| SMILES | CN1C(=O)c2ccc(OC(=O)CCc3ccc(N)nc3)cc2C1=O |
|---|
| Structure | 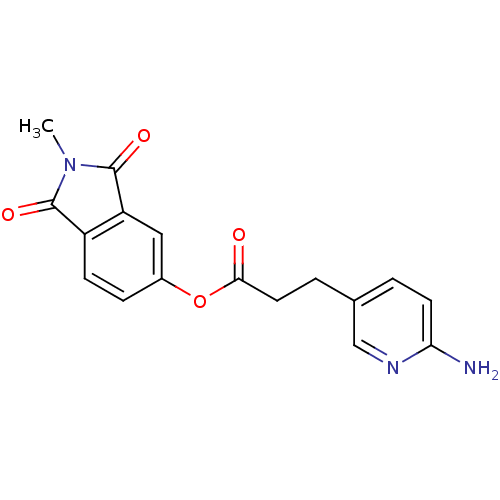
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Aoyama, H; Ijuin, R; Kato, JY; Urushiyama, S; Tetsuhashi, M; Hashimoto, Y; Yokomatsu, T Discovery of non-competitive thrombin inhibitor derived from competitive tryptase inhibitor skeleton: Shift in molecular recognition resulted from skeletal conversion of carboxylate into phosphonate. Bioorg Med Chem Lett25:3676-80 (2015) [PubMed] Article
Aoyama, H; Ijuin, R; Kato, JY; Urushiyama, S; Tetsuhashi, M; Hashimoto, Y; Yokomatsu, T Discovery of non-competitive thrombin inhibitor derived from competitive tryptase inhibitor skeleton: Shift in molecular recognition resulted from skeletal conversion of carboxylate into phosphonate. Bioorg Med Chem Lett25:3676-80 (2015) [PubMed] Article