| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM474233 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Binding Affinities to Different Adenosine Receptors |
|---|
| IC50 | 0.700±n/a nM |
|---|
| Citation |  Zeng, Q; Qi, C; Tsui, H; Yang, Z; Zhang, X Triazolo-pyrimidine compounds and uses thereof US Patent US10858365 Publication Date 12/8/2020 Zeng, Q; Qi, C; Tsui, H; Yang, Z; Zhang, X Triazolo-pyrimidine compounds and uses thereof US Patent US10858365 Publication Date 12/8/2020 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | A2A adenosine receptor (hA2A) | AA2AR_HUMAN | ADENOSINE A2 | ADENOSINE A2a | ADORA2 | ADORA2A | Adenosine A2A receptor (A2AAR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 44716.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29274 |
|---|
| Residue: | 412 |
|---|
| Sequence: | MPIMGSSVYITVELAIAVLAILGNVLVCWAVWLNSNLQNVTNYFVVSLAAADIAVGVLAI
PFAITISTGFCAACHGCLFIACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGTR
AKGIIAICWVLSFAIGLTPMLGWNNCGQPKEGKNHSQGCGEGQVACLFEDVVPMNYMVYF
NFFACVLVPLLLMLGVYLRIFLAARRQLKQMESQPLPGERARSTLQKEVHAAKSLAIIVG
LFALCWLPLHIINCFTFFCPDCSHAPLWLMYLAIVLSHTNSVVNPFIYAYRIREFRQTFR
KIIRSHVLRQQEPFKAAGTSARVLAAHGSDGEQVSLRLNGHPPGVWANGSAPHPERRPNG
YALGLVSGGSAQESQGNTGLPDVELLSHELKGVCPEPPGLDDPLAQDGAGVS
|
|
|
|---|
| BDBM474233 |
|---|
| n/a |
|---|
| Name | BDBM474233 |
|---|
| Synonyms: | 5-[5-amino-7-(4-fluorophenyl)-2-[(3-fluoropyrid in-2-yl)methyl]-[1,2,4]triazolo[1,5-c]pyrimidin-8- yl]-l-(propan-2-yl)-l,2-dihydropyridin-2-one | US10858365, Compound 55 | US11629147, Cmpd. 55 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H21F2N7O |
|---|
| Mol. Mass. | 473.4773 |
|---|
| SMILES | CC(C)n1cc(ccc1=O)-c1c(nc(N)n2nc(Cc3ncccc3F)nc12)-c1ccc(F)cc1 |
|---|
| Structure | 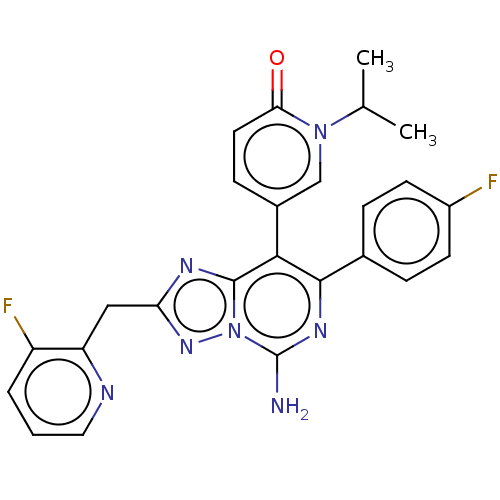
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zeng, Q; Qi, C; Tsui, H; Yang, Z; Zhang, X Triazolo-pyrimidine compounds and uses thereof US Patent US10858365 Publication Date 12/8/2020
Zeng, Q; Qi, C; Tsui, H; Yang, Z; Zhang, X Triazolo-pyrimidine compounds and uses thereof US Patent US10858365 Publication Date 12/8/2020