

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Adenosine receptor A2b | ||
| Ligand | BDBM561411 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Adenosine A2B Receptor Cyclic AMP GS Assay | ||
| EC50 | <10±n/a nM | ||
| Citation |  Huang, T; Wang, X Pyrazolopyridines and triazolopyridines as A2A / A2B inhibitors US Patent US11390624 Publication Date 7/19/2022 Huang, T; Wang, X Pyrazolopyridines and triazolopyridines as A2A / A2B inhibitors US Patent US11390624 Publication Date 7/19/2022 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Adenosine receptor A2b | |||
| Name: | Adenosine receptor A2b | ||
| Synonyms: | AA2BR_HUMAN | ADENOSINE A2B | ADORA2B | Adenosine receptor A2B (A2B) | Adenosine receptors A2b | Adenosine receptors; A2a & A2b | ||
| Type: | G Protein-Coupled Receptor (GPCR) | ||
| Mol. Mass.: | 36341.22 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | n/a | ||
| Residue: | 332 | ||
| Sequence: |
| ||
| BDBM561411 | |||
| n/a | |||
| Name | BDBM561411 | ||
| Synonyms: | 3-(4-amino-2-((6-methylpyridin-2-yl)methyl)-7-(3-methylpyridin-4-yl)-2H-[1,2,3]triazolo[4,5-c]pyridin-6-yl)benzonitrile | US11390624, Example 26 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C25H20N8 | ||
| Mol. Mass. | 432.4799 | ||
| SMILES | Cc1cccc(Cn2nc3c(N)nc(-c4cccc(c4)C#N)c(-c4ccncc4C)c3n2)n1 |(8.93,1.13,;7.39,1.13,;6.62,2.46,;5.08,2.46,;4.31,1.13,;5.08,-.21,;4.31,-1.54,;2.77,-1.54,;1.87,-2.79,;.4,-2.31,;-.93,-3.08,;-.93,-4.62,;-2.26,-2.31,;-2.26,-.77,;-3.6,,;-3.6,1.54,;-4.93,2.31,;-6.27,1.54,;-6.27,,;-4.93,-.77,;-7.6,-.77,;-8.93,-1.54,;-.93,,;-.93,1.54,;-2.26,2.31,;-2.26,3.85,;-.93,4.62,;.4,3.85,;.4,2.31,;1.74,1.54,;.4,-.77,;1.87,-.29,;6.62,-.21,)| | ||
| Structure | 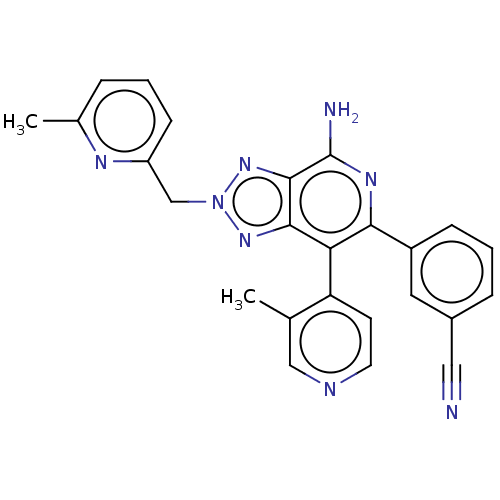
| ||