

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Serine/threonine-protein kinase/endoribonuclease IRE1 | ||
| Ligand | BDBM627502 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | Inhibition of Kinase Activity of IRE1alpha | ||
| IC50 | 2.70±n/a nM | ||
| Citation |  Keenan, R; Sutton, J; Hynd, G; Panchal, T Pyrazolopyridine Compounds and Methods of Inhibiting IRE1 Using Same US Patent US20230331719 Publication Date 10/19/2023 Keenan, R; Sutton, J; Hynd, G; Panchal, T Pyrazolopyridine Compounds and Methods of Inhibiting IRE1 Using Same US Patent US20230331719 Publication Date 10/19/2023 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Serine/threonine-protein kinase/endoribonuclease IRE1 | |||
| Name: | Serine/threonine-protein kinase/endoribonuclease IRE1 | ||
| Synonyms: | ERN1 | ERN1_HUMAN | Endoplasmic reticulum-to-nucleus signaling 1 | Endoribonuclease | IRE1 | IRE1a | Inositol requiring enzyme 1 (IRE-1alpha) | Inositol-requiring enzyme 1 (IRE1a) | Inositol-requiring protein 1 | Inositol-requiring protein 1 (IRE1a) | Ire1-alpha | Serine/threonine-protein kinase | Serine/threonine-protein kinase/endoribonuclease IRE1 | Serine/threonine-protein kinase/endoribonuclease IRE1 Alpha | hIRE1p | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 109731.20 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | O75460 | ||
| Residue: | 977 | ||
| Sequence: |
| ||
| BDBM627502 | |||
| n/a | |||
| Name | BDBM627502 | ||
| Synonyms: | N-(4-(4-amino-1-isopropyl-7-((1r,4r)-4-((2-methoxyethyl)amino)cyclohexyl)-1H-pyrazolo[4,3-c]pyridin-3-yl)-2,5-difluorophenyl)-2-chloro-5-(difluoromethoxy)benzenesulfonamide | US20230331719, Compound A1 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C31H35ClF4N6O4S | ||
| Mol. Mass. | 699.159 | ||
| SMILES | COCCN[C@H]1CC[C@@H](CC1)c1cnc(N)c2c(nn(C(C)C)c12)-c1cc(F)c(NS(=O)(=O)c2cc(OC(F)F)ccc2Cl)cc1F |r,wU:8.11,wD:5.4,(-4.26,-12.54,;-4.26,-11,;-2.93,-10.23,;-2.93,-8.69,;-4.26,-7.92,;-4.26,-6.38,;-2.93,-5.61,;-2.93,-4.07,;-4.26,-3.3,;-5.59,-4.07,;-5.59,-5.61,;-4.26,-1.76,;-5.59,-.99,;-5.59,.55,;-4.26,1.32,;-4.26,2.86,;-2.93,.55,;-1.46,1.02,;-.56,-.22,;-1.46,-1.47,;-.99,-2.93,;-2.02,-4.08,;.52,-3.25,;-2.93,-.99,;-.99,2.49,;-2.02,3.63,;-1.54,5.1,;-2.57,6.24,;-.03,5.42,;.44,6.88,;1.95,7.2,;1.55,8.69,;3.04,8.29,;2.98,6.06,;2.5,4.59,;3.53,3.45,;3.06,1.99,;4.09,.84,;3.61,-.62,;5.59,1.16,;5.04,3.77,;5.52,5.23,;4.48,6.38,;4.96,7.84,;1,4.27,;.52,2.81,;1.55,1.67,)| | ||
| Structure | 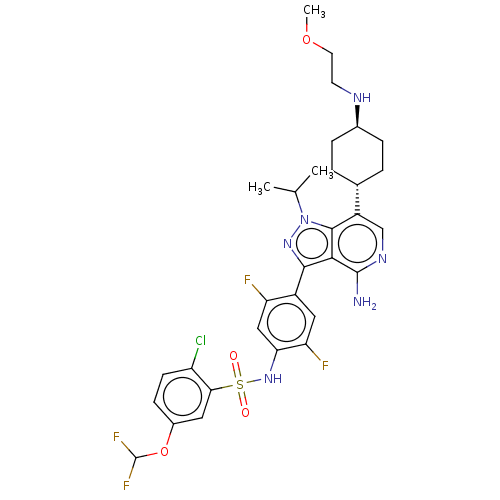
| ||