| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Nucleoprotein |
|---|
| Ligand | BDBM614513 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | TBD |
|---|
| EC50 | 20.0±n/a nM |
|---|
| Citation |  BARRETT, M; COCKERILL, GS; GOOD, J; AVERY, CA; COCHRANE, EJ; JONES, SP; ONIONS, ST; WARNER, AJ BENZODIAZEPINE DERIVATIVES USEFUL IN TREATING A RESPIRATORY SYNCYTIAL VIRUS INFECTION US Patent US20230270751 Publication Date 8/31/2023 BARRETT, M; COCKERILL, GS; GOOD, J; AVERY, CA; COCHRANE, EJ; JONES, SP; ONIONS, ST; WARNER, AJ BENZODIAZEPINE DERIVATIVES USEFUL IN TREATING A RESPIRATORY SYNCYTIAL VIRUS INFECTION US Patent US20230270751 Publication Date 8/31/2023 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Nucleoprotein |
|---|
| Name: | Nucleoprotein |
|---|
| Synonyms: | N | NCAP_HRSVA | Nucleocapsid protein | Protein N |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 43455.45 |
|---|
| Organism: | Human respiratory syncytial virus A (strain A2) |
|---|
| Description: | ChEMBL_117398 |
|---|
| Residue: | 391 |
|---|
| Sequence: | MALSKVKLNDTLNKDQLLSSSKYTIQRSTGDSIDTPNYDVQKHINKLCGMLLITEDANHK
FTGLIGMLYAMSRLGREDTIKILRDAGYHVKANGVDVTTHRQDINGKEMKFEVLTLASLT
TEIQINIEIESRKSYKKMLKEMGEVAPEYRHDSPDCGMIILCIAALVITKLAAGDRSGLT
AVIRRANNVLKNEMKRYKGLLPKDIANSFYEVFEKHPHFIDVFVHFGIAQSSTRGGSRVE
GIFAGLFMNAYGAGQVMLRWGVLAKSVKNIMLGHASVQAEMEQVVEVYEYAQKLGGEAGF
YHILNNPKASLLSLTQFPHFSSVVLGNAAGLGIMGEYRGTPRNQDLYDAAKAYAEQLKEN
GVINYSVLDLTAEELEAIKHQLNPKDNDVEL
|
|
|
|---|
| BDBM614513 |
|---|
| n/a |
|---|
| Name | BDBM614513 |
|---|
| Synonyms: | (5R)-2-[2-Fluoro-6-[(1R,4R)-2-oxa-5-azabicyclo[2.2.1]heptan-5-yl]pyridin-3-yl]-5-methyl-N-[(3S)-9-fluoro-2-oxo-5-phenyl-1,3-dihydro-1,4-benzodiazepin-3-yl]-6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxamide | US20230270751, Example 90 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H29F2N7O4 |
|---|
| Mol. Mass. | 625.6247 |
|---|
| SMILES | C[C@@H]1CCn2nc(c(C(=O)N[C@H]3N=C(c4ccccc4)c4cccc(F)c4NC3=O)c2O1)-c1ccc(nc1F)N1C[C@H]2C[C@@H]1CO2 |t:12| |
|---|
| Structure | 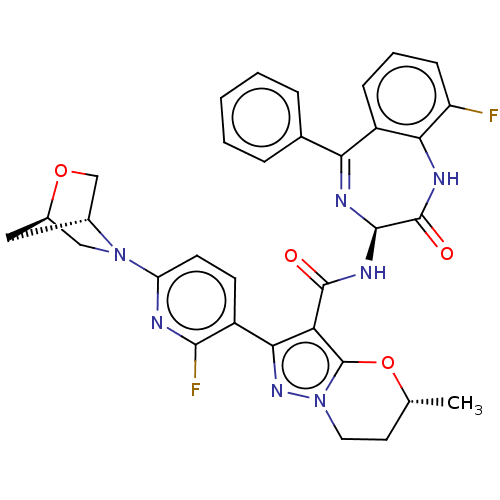
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 BARRETT, M; COCKERILL, GS; GOOD, J; AVERY, CA; COCHRANE, EJ; JONES, SP; ONIONS, ST; WARNER, AJ BENZODIAZEPINE DERIVATIVES USEFUL IN TREATING A RESPIRATORY SYNCYTIAL VIRUS INFECTION US Patent US20230270751 Publication Date 8/31/2023
BARRETT, M; COCKERILL, GS; GOOD, J; AVERY, CA; COCHRANE, EJ; JONES, SP; ONIONS, ST; WARNER, AJ BENZODIAZEPINE DERIVATIVES USEFUL IN TREATING A RESPIRATORY SYNCYTIAL VIRUS INFECTION US Patent US20230270751 Publication Date 8/31/2023