

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Lysosomal acid glucosylceramidase | ||
| Ligand | BDBM313452 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | High Throughput Screening (HTS) by Fluorescence Polarization (pH = 7.2) | ||
| Kd | 126±0 nM | ||
| Citation |  Krainc, D; Silverman, RB; Zheng, J Substituted quinazoline compounds and uses thereof for modulating glucocerebrosidase activity US Patent US10167270 Publication Date 1/1/2019 Krainc, D; Silverman, RB; Zheng, J Substituted quinazoline compounds and uses thereof for modulating glucocerebrosidase activity US Patent US10167270 Publication Date 1/1/2019 | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Lysosomal acid glucosylceramidase | |||
| Name: | Lysosomal acid glucosylceramidase | ||
| Synonyms: | Acid beta-glucosidase | Alglucerase | Beta-glucocerebrosidase | Beta-glucocerebrosidase (GC) | D-glucosyl-N-acylsphingosine glucohydrolase | GBA | GBA1 | GBA1_HUMAN | GC | GCase | GLUC | Glucocerebrosidase (GBA) | Glucosylceramidase (GBA) | Glucosylceramidase (GCase) | Glucosylceramidase precursor (Beta-glucocerebrosidase) (Acid beta-glucosidase) (D-glucosyl-N-acylsphingosine glucohydrolase) (Alglucerase) (Imiglucerase) | Imiglucerase | beta-glucocerebrosidase (GCase) | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 59724.64 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | The beta-Glu activity was measured with commercially available beta-glucocerebrosidase (Ceredase) as the enzyme source. | ||
| Residue: | 536 | ||
| Sequence: |
| ||
| BDBM313452 | |||
| n/a | |||
| Name | BDBM313452 | ||
| Synonyms: | US10167270, Example 159 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C54H52N7O7 | ||
| Mol. Mass. | 911.0328 | ||
| SMILES | CN(C)c1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCOCCOCC(=O)Nc3ccc(cc3)-c3nc(NC4Cc5ccccc5C4)c4ccccc4n3)c3ccc(cc3oc2c1)=[N+](C)C |(12.67,-5.32,;11.34,-4.55,;10,-5.32,;11.34,-3.01,;10,-2.24,;10,-.7,;11.34,.07,;11.34,1.61,;10,2.38,;8.67,1.61,;7.34,2.38,;7.34,3.92,;8.67,4.69,;10.05,3.95,;11.38,4.72,;12.89,4.4,;11.38,6.26,;6,4.69,;6,6.23,;4.67,3.92,;3.33,4.69,;2,3.92,;.67,4.69,;-.67,3.92,;-2,4.69,;-3.33,3.92,;-4.67,4.69,;-6,3.92,;-6,2.38,;-7.34,4.69,;-8.67,3.92,;-10,4.69,;-11.34,3.92,;-11.34,2.38,;-10,1.61,;-8.67,2.38,;-12.67,1.61,;-12.67,.07,;-14,-.7,;-14,-2.24,;-12.67,-3.01,;-12.67,-4.55,;-11.21,-5.02,;-10.58,-6.43,;-9.05,-6.59,;-8.14,-5.34,;-8.77,-3.94,;-10.3,-3.78,;-11.21,-2.53,;-15.34,.07,;-16.67,-.7,;-18,.07,;-18,1.61,;-16.67,2.38,;-15.34,1.61,;-14,2.38,;12.67,2.38,;12.67,3.92,;14,4.69,;15.34,3.92,;15.34,2.38,;14,1.61,;14,.07,;12.67,-.7,;12.67,-2.24,;16.67,4.69,;18,3.92,;16.67,6.23,)| | ||
| Structure | 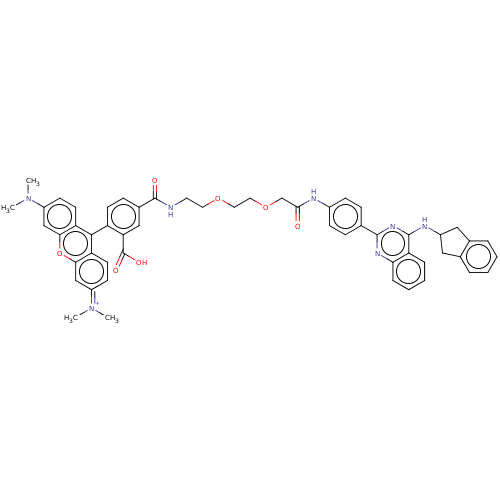
| ||