| Reaction Details |
|---|
 | Report a problem with these data |
| Target | cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A |
|---|
| Ligand | BDBM50425125 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | In Vitro Assay PDE10A |
|---|
| pH | 7.8±n/a |
|---|
| Temperature | 298.15±n/a K |
|---|
| IC50 | 160±n/a nM |
|---|
| Comments | extracted |
|---|
| Citation |  Megens, AA; Langlois, XJ; Andrés-Gil, JI Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl-[1,2,4]triazolo-[4,3-A]]quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological of metabolic disorders US Patent US9669035 Publication Date 6/6/2017 Megens, AA; Langlois, XJ; Andrés-Gil, JI Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl-[1,2,4]triazolo-[4,3-A]]quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological of metabolic disorders US Patent US9669035 Publication Date 6/6/2017 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A |
|---|
| Name: | cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A |
|---|
| Synonyms: | PDE10_RAT | Pde10a | Phosphodiesterase 10 | Phosphodiesterase 10A (PDE10A2) | Phosphodiesterase Type 10 (PDE10A) | Rat recombinant PDE10a (rPDE10a) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 90160.88 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | PDE10A was generated from the full-length recombinant rat clone transfected into Sf9 cells. |
|---|
| Residue: | 794 |
|---|
| Sequence: | MEDGPSNNASCFRRLTECFLSPSLTDEKVKAYLSLHPQVLDEFVSESVSAETVEKWLKRK
NNKAEDEPSPKEVSRYQDTNMQGVVYELNSYIEQRLDTGGDNHLLLYELSSIIRIATKAD
GFALYFLGECNNSLCVFTPPGMKEGQPRLIPAGPITQGTTISAYVAKSRKTLLVEDILGD
ERFPRGTGLESGTRIQSVLCLPIVTAIGDLIGILELYRHWGKEAFCLSHQEVATANLAWA
SVAIHQVQVCRGLAKQTELNDFLLDVSKTYFDNIVAIDSLLEHIMIYAKNLVNADRCALF
QVDHKNKELYSDLFDIGEEKEGKPVFKKTKEIRFSIEKGIAGQVARTGEVLNIPDAYADP
RFNREVDLYTGYTTRNILCMPIVSRGSVIGVVQMVNKISGSAFSKTDENNFKMFAVFCAL
ALHCANMYHRIRHSECIYRVTMEKLSYHSICTSEEWQGLMHFNLPARICRDIELFHFDIG
PFENMWPGIFVYMIHRSCGTSCFELEKLCRFIMSVKKNYRRVPYHNWKHAVTVAHCMYAI
LQNNNGLFTDLERKGLLIACLCHDLDHRGFSNSYLQKFDHPLAALYSTSTMEQHHFSQTV
SILQLEGHNIFSTLSSSEYEQVLEIIRKAIIATDLALYFGNRKQLEEMYQTGSLNLHNQS
HRDRVIGLMMTACDLCSVTKLWPVTKLTANDIYAEFWAEGDEMKKLGIQPIPMMDRDKRD
EVPQGQLGFYNAVAIPCYTTLTQILPPTEPLLKACRDNLNQWEKVIRGEETAMWISGPAT
SKSTSEKPTRKVDD
|
|
|
|---|
| BDBM50425125 |
|---|
| n/a |
|---|
| Name | BDBM50425125 |
|---|
| Synonyms: | CHEMBL2313232 | US9669035, B-4 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H18ClN5 |
|---|
| Mol. Mass. | 351.833 |
|---|
| SMILES | CCNCc1ccc2nc(C)c3nnc(-c4ccccc4Cl)n3c2c1 |
|---|
| Structure | 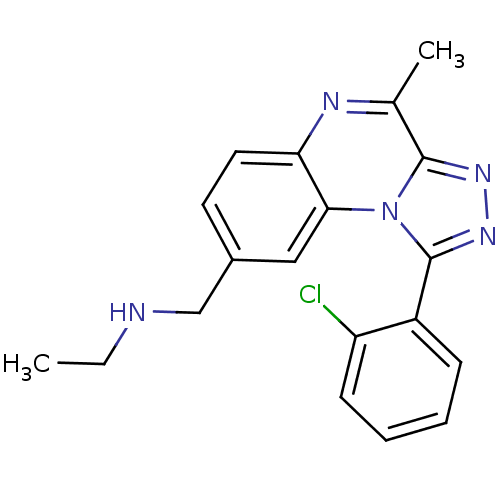
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Megens, AA; Langlois, XJ; Andrés-Gil, JI Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl-[1,2,4]triazolo-[4,3-A]]quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological of metabolic disorders US Patent US9669035 Publication Date 6/6/2017
Megens, AA; Langlois, XJ; Andrés-Gil, JI Combinations comprising PDE 2 inhibitors such as 1-aryl-4-methyl-[1,2,4]triazolo-[4,3-A]]quinoxaline compounds and PDE 10 inhibitors for use in the treatment of neurological of metabolic disorders US Patent US9669035 Publication Date 6/6/2017