| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Mineralocorticoid receptor |
|---|
| Ligand | BDBM19214 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Mineralocorticoid Receptor Transactivation Potencies for Cortisol and 17-ester Derivatives |
|---|
| EC50 | 0.470±n/a nM |
|---|
| Citation |  Edelman, JL; Nehme, A Pharmaceutical compositions and methods of use of 4-pregenen-11β-17-21-triol-3,20-dione derivatives US Patent US10188667 Publication Date 1/29/2019 Edelman, JL; Nehme, A Pharmaceutical compositions and methods of use of 4-pregenen-11β-17-21-triol-3,20-dione derivatives US Patent US10188667 Publication Date 1/29/2019 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Mineralocorticoid receptor |
|---|
| Name: | Mineralocorticoid receptor |
|---|
| Synonyms: | MCR | MCR_HUMAN | MLR | MR | NR3C2 | Nuclear receptor subfamily 3 group C member 2 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 107076.42 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P08235 |
|---|
| Residue: | 984 |
|---|
| Sequence: | METKGYHSLPEGLDMERRWGQVSQAVERSSLGPTERTDENNYMEIVNVSCVSGAIPNNST
QGSSKEKQELLPCLQQDNNRPGILTSDIKTELESKELSATVAESMGLYMDSVRDADYSYE
QQNQQGSMSPAKIYQNVEQLVKFYKGNGHRPSTLSCVNTPLRSFMSDSGSSVNGGVMRAV
VKSPIMCHEKSPSVCSPLNMTSSVCSPAGINSVSSTTASFGSFPVHSPITQGTPLTCSPN
VENRGSRSHSPAHASNVGSPLSSPLSSMKSSISSPPSHCSVKSPVSSPNNVTLRSSVSSP
ANINNSRCSVSSPSNTNNRSTLSSPAASTVGSICSPVNNAFSYTASGTSAGSSTLRDVVP
SPDTQEKGAQEVPFPKTEEVESAISNGVTGQLNIVQYIKPEPDGAFSSSCLGGNSKINSD
SSFSVPIKQESTKHSCSGTSFKGNPTVNPFPFMDGSYFSFMDDKDYYSLSGILGPPVPGF
DGNCEGSGFPVGIKQEPDDGSYYPEASIPSSAIVGVNSGGQSFHYRIGAQGTISLSRSAR
DQSFQHLSSFPPVNTLVESWKSHGDLSSRRSDGYPVLEYIPENVSSSTLRSVSTGSSRPS
KICLVCGDEASGCHYGVVTCGSCKVFFKRAVEGQHNYLCAGRNDCIIDKIRRKNCPACRL
QKCLQAGMNLGARKSKKLGKLKGIHEEQPQQQQPPPPPPPPQSPEEGTTYIAPAKEPSVN
TALVPQLSTISRALTPSPVMVLENIEPEIVYAGYDSSKPDTAENLLSTLNRLAGKQMIQV
VKWAKVLPGFKNLPLEDQITLIQYSWMCLSSFALSWRSYKHTNSQFLYFAPDLVFNEEKM
HQSAMYELCQGMHQISLQFVRLQLTFEEYTIMKVLLLLSTIPKDGLKSQAAFEEMRTNYI
KELRKMVTKCPNNSGQSWQRFYQLTKLLDSMHDLVSDLLEFCFYTFRESHALKVEFPAML
VEIISDQLPKVESGNAKPLYFHRK
|
|
|
|---|
| BDBM19214 |
|---|
| n/a |
|---|
| Name | BDBM19214 |
|---|
| Synonyms: | (1S,2R,10S,11S,14S,15R,17S)-17-hydroxy-14-(2-hydroxyacetyl)-2-methyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-ene-15-carbaldehyde | Aldosterone | US10188667, Aldosterone | [3H]Aldosterone |
|---|
| Type | Steroid |
|---|
| Emp. Form. | C21H28O5 |
|---|
| Mol. Mass. | 360.444 |
|---|
| SMILES | [H][C@@]12CC[C@H](C(=O)CO)[C@]1(C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C)C=O |t:21| |
|---|
| Structure | 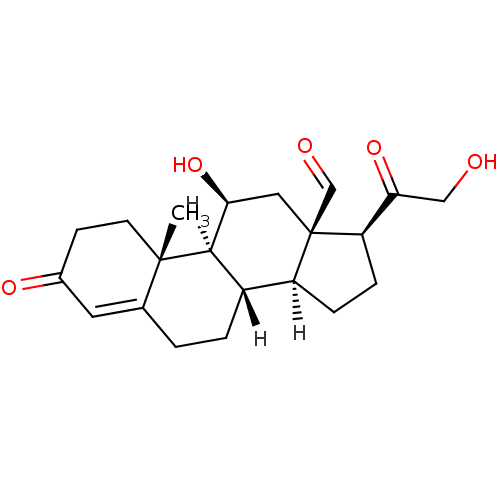
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Edelman, JL; Nehme, A Pharmaceutical compositions and methods of use of 4-pregenen-11β-17-21-triol-3,20-dione derivatives US Patent US10188667 Publication Date 1/29/2019
Edelman, JL; Nehme, A Pharmaceutical compositions and methods of use of 4-pregenen-11β-17-21-triol-3,20-dione derivatives US Patent US10188667 Publication Date 1/29/2019