| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine protease 1 |
|---|
| Ligand | BDBM14072 |
|---|
| Substrate/Competitor | BDBM14141 |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| Ki | 22±n/a nM |
|---|
| Citation |  Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Serine protease 1 |
|---|
| Name: | Serine protease 1 |
|---|
| Synonyms: | Beta-Trypsin | Cationic trypsin | PRSS1 | TRP1 | TRY1 | TRY1_BOVIN | TRYP1 | Trypsin | Trypsin I |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 25790.52 |
|---|
| Organism: | Bos taurus (bovine) |
|---|
| Description: | P00760 |
|---|
| Residue: | 246 |
|---|
| Sequence: | MKTFIFLALLGAAVAFPVDDDDKIVGGYTCGANTVPYQVSLNSGYHFCGGSLINSQWVVS
AAHCYKSGIQVRLGEDNINVVEGNEQFISASKSIVHPSYNSNTLNNDIMLIKLKSAASLN
SRVASISLPTSCASAGTQCLISGWGNTKSSGTSYPDVLKCLKAPILSDSSCKSAYPGQIT
SNMFCAGYLEGGKDSCQGDSGGPVVCSGKLQGIVSWGSGCAQKNKPGVYTKVCNYVSWIK
QTIASN
|
|
|
|---|
| BDBM14072 |
|---|
| BDBM14141 |
|---|
| Name | BDBM14072 |
|---|
| Synonyms: | 2-ketobenzothiazole 12 | 2-{[(2R)-1-[(2S)-2-{[1-(1,3-benzothiazol-2-yl)-5-carbamimidamido-1-oxopentan-2-yl]carbamoyl}pyrrolidin-1-yl]-3-cyclohexyl-1-oxopropan-2-yl]amino}acetic acid |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H41N7O5S |
|---|
| Mol. Mass. | 599.745 |
|---|
| SMILES | [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6](-[#8])=O)-[#6](=O)-c1nc2ccccc2s1 |r| |
|---|
| Structure | 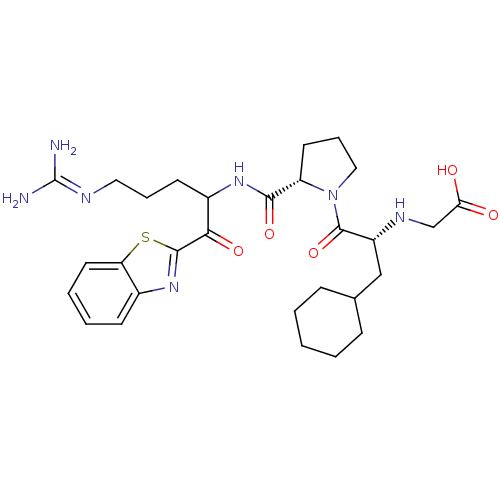
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article
Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article