| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin S |
|---|
| Ligand | BDBM19855 |
|---|
| Substrate/Competitor | BDBM19490 |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| IC50 | 6350±n/a nM |
|---|
| EC50 | 480±n/a nM |
|---|
| Citation |  Falgueyret, JP; Desmarais, S; Oballa, R; Black, WC; Cromlish, W; Khougaz, K; Lamontagne, S; Massé, F; Riendeau, D; Toulmond, S; Percival, MD Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem48:7535-43 (2005) [PubMed] Article Falgueyret, JP; Desmarais, S; Oballa, R; Black, WC; Cromlish, W; Khougaz, K; Lamontagne, S; Massé, F; Riendeau, D; Toulmond, S; Percival, MD Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem48:7535-43 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Cathepsin S |
|---|
| Name: | Cathepsin S |
|---|
| Synonyms: | CATS_MOUSE | Cats | Ctss |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 38476.67 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | O70370 |
|---|
| Residue: | 340 |
|---|
| Sequence: | MRAPGHAAIRWLFWMPLVCSVAMEQLQRDPTLDYHWDLWKKTHEKEYKDKNEEEVRRLIW
EKNLKFIMIHNLEYSMGMHTYQVGMNDMGDMTNEEILCRMGALRIPRQSPKTVTFRSYSN
RTLPDTVDWREKGCVTEVKYQGSCGACWAFSAVGALEGQLKLKTGKLISLSAQNLVDCSN
EEKYGNKGCGGGYMTEAFQYIIDNGGIEADASYPYKATDEKCHYNSKNRAATCSRYIQLP
FGDEDALKEAVATKGPVSVGIDASHSSFFFYKSGVYDDPSCTGNVNHGVLVVGYGTLDGK
DYWLVKNSWGLNFGDQGYIRMARNNKNHCGIASYCSYPEI
|
|
|
|---|
| BDBM19855 |
|---|
| BDBM19490 |
|---|
| Name | BDBM19855 |
|---|
| Synonyms: | Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbamoyl)cyclohexyl]-4-(4-propylpiperazin-1-yl) | N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-(4-propylpiperazin-1-yl)benzamide | basic piperazine-containing compound, 10 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H33N5O2 |
|---|
| Mol. Mass. | 411.5404 |
|---|
| SMILES | CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N |
|---|
| Structure | 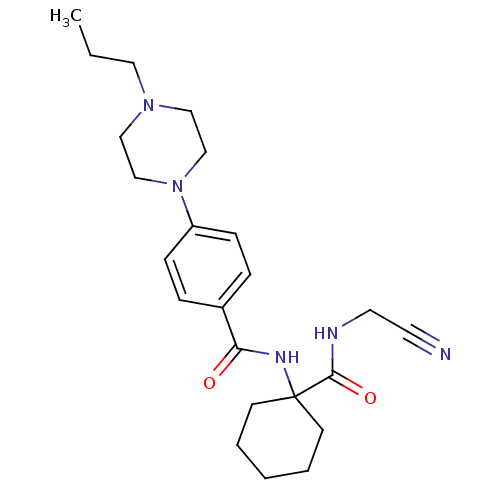
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Falgueyret, JP; Desmarais, S; Oballa, R; Black, WC; Cromlish, W; Khougaz, K; Lamontagne, S; Massé, F; Riendeau, D; Toulmond, S; Percival, MD Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem48:7535-43 (2005) [PubMed] Article
Falgueyret, JP; Desmarais, S; Oballa, R; Black, WC; Cromlish, W; Khougaz, K; Lamontagne, S; Massé, F; Riendeau, D; Toulmond, S; Percival, MD Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem48:7535-43 (2005) [PubMed] Article