| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-hexosaminidase subunit beta |
|---|
| Ligand | BDBM50182804 |
|---|
| Substrate/Competitor | BDBM18365 |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| pH | 4.25±0 |
|---|
| Temperature | 303.15±0 K |
|---|
| Ki | 5.4e+2± 2.5e+2 nM |
|---|
| Citation |  Ho, CW; Popat, SD; Liu, TW; Tsai, KC; Ho, MJ; Chen, WH; Yang, AS; Lin, CH Development of GlcNAc-inspired iminocyclitiols as potent and selective N-acetyl-beta-hexosaminidase inhibitors. ACS Chem Biol5:489-97 (2010) [PubMed] Article Ho, CW; Popat, SD; Liu, TW; Tsai, KC; Ho, MJ; Chen, WH; Yang, AS; Lin, CH Development of GlcNAc-inspired iminocyclitiols as potent and selective N-acetyl-beta-hexosaminidase inhibitors. ACS Chem Biol5:489-97 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Beta-hexosaminidase subunit beta |
|---|
| Name: | Beta-hexosaminidase subunit beta |
|---|
| Synonyms: | Beta-N-acetylhexosaminidase subunit beta | Beta-hexosaminidase subunit beta | Beta-hexosaminidase subunit beta (Hex B) | HEXB | HEXB_HUMAN | Hexosaminidase subunit B |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 63112.58 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P07686 |
|---|
| Residue: | 556 |
|---|
| Sequence: | MELCGLGLPRPPMLLALLLATLLAAMLALLTQVALVVQVAEAARAPSVSAKPGPALWPLP
LLVKMTPNLLHLAPENFYISHSPNSTAGPSCTLLEEAFRRYHGYIFGFYKWHHEPAEFQA
KTQVQQLLVSITLQSECDAFPNISSDESYTLLVKEPVAVLKANRVWGALRGLETFSQLVY
QDSYGTFTINESTIIDSPRFSHRGILIDTSRHYLPVKIILKTLDAMAFNKFNVLHWHIVD
DQSFPYQSITFPELSNKGSYSLSHVYTPNDVRMVIEYARLRGIRVLPEFDTPGHTLSWGK
GQKDLLTPCYSRQNKLDSFGPINPTLNTTYSFLTTFFKEISEVFPDQFIHLGGDEVEFKC
WESNPKIQDFMRQKGFGTDFKKLESFYIQKVLDIIATINKGSIVWQEVFDDKAKLAPGTI
VEVWKDSAYPEELSRVTASGFPVILSAPWYLDLISYGQDWRKYYKVEPLDFGGTQKQKQL
FIGGEACLWGEYVDATNLTPRLWPRASAVGERLWSSKDVRDMDDAYDRLTRHRCRMVERG
IAAQPLYAGYCNHENM
|
|
|
|---|
| BDBM50182804 |
|---|
| BDBM18365 |
|---|
| Name | BDBM50182804 |
|---|
| Synonyms: | 2-ACETAMIDO-1,2-DIDEOXYNOJIRMYCIN | CHEMBL382689 | Iminocyclitol, 5 | N-((3S,4R,5R,6R)-4,5-dihydroxy-6-(hydroxymethyl)piperidin-3-yl)acetamide | N-[4,5-dihydroxy-6-(hydroxymethyl)piperidin-3-yl]acetamide, 1 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H16N2O4 |
|---|
| Mol. Mass. | 204.2236 |
|---|
| SMILES | CC(=O)N[C@H]1CN[C@H](CO)[C@@H](O)[C@@H]1O |
|---|
| Structure | 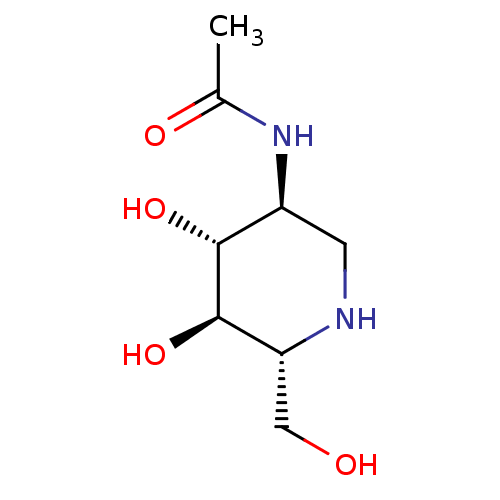
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ho, CW; Popat, SD; Liu, TW; Tsai, KC; Ho, MJ; Chen, WH; Yang, AS; Lin, CH Development of GlcNAc-inspired iminocyclitiols as potent and selective N-acetyl-beta-hexosaminidase inhibitors. ACS Chem Biol5:489-97 (2010) [PubMed] Article
Ho, CW; Popat, SD; Liu, TW; Tsai, KC; Ho, MJ; Chen, WH; Yang, AS; Lin, CH Development of GlcNAc-inspired iminocyclitiols as potent and selective N-acetyl-beta-hexosaminidase inhibitors. ACS Chem Biol5:489-97 (2010) [PubMed] Article