| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase |
|---|
| Ligand | BDBM403547 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Measurement of Human MGAT2 Inhibitory Activity |
|---|
| IC50 | 0.620±n/a nM |
|---|
| Citation |  Nishiura, Y; Kurahashi, K; Ohyabu, N; Sasaki, Y Non-aromatic heterocyclic derivative having MGAT2 inhibitory activity US Patent US10335401 Publication Date 7/2/2019 Nishiura, Y; Kurahashi, K; Ohyabu, N; Sasaki, Y Non-aromatic heterocyclic derivative having MGAT2 inhibitory activity US Patent US10335401 Publication Date 7/2/2019 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase |
|---|
| Name: | Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase |
|---|
| Synonyms: | 2.4.1.143 | Beta-1,2-N-acetylglucosaminyltransferase II | GNT-II | GlcNAc-T II | MGAT2 | MGAT2_HUMAN | Mannoside acetylglucosaminyltransferase 2 | Monoacylglycerol acyltransferase 2 (MGAT2) | Monoacylglycerol acyltransferase type 2 (h-MGAT2) | N-glycosyl-oligosaccharide-glycoprotein N-acetylglucosaminyltransferase II | h-MGAT2 (human monoacylglycerol acyltransferase type 2) |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 51567.80 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q10469 |
|---|
| Residue: | 447 |
|---|
| Sequence: | MRFRIYKRKVLILTLVVAACGFVLWSSNGRQRKNEALAPPLLDAEPARGAGGRGGDHPSV
AVGIRRVSNVSAASLVPAVPQPEADNLTLRYRSLVYQLNFDQTLRNVDKAGTWAPRELVL
VVQVHNRPEYLRLLLDSLRKAQGIDNVLVIFSHDFWSTEINQLIAGVNFCPVLQVFFPFS
IQLYPNEFPGSDPRDCPRDLPKNAALKLGCINAEYPDSFGHYREAKFSQTKHHWWWKLHF
VWERVKILRDYAGLILFLEEDHYLAPDFYHVFKKMWKLKQQECPECDVLSLGTYSASRSF
YGMADKVDVKTWKSTEHNMGLALTRNAYQKLIECTDTFCTYDDYNWDWTLQYLTVSCLPK
FWKVLVPQIPRIFHAGDCGMHHKKTCRPSTQSAQIESLLNNNKQYMFPETLTISEKFTVV
AISPPRKNGGWGDIRDHELCKSYRRLQ
|
|
|
|---|
| BDBM403547 |
|---|
| n/a |
|---|
| Name | BDBM403547 |
|---|
| Synonyms: | US10335401, No. I-57 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H25F8N3O6S |
|---|
| Mol. Mass. | 659.545 |
|---|
| SMILES | CS(=O)(=O)CC(=O)NCC1=C(C[C@](NC1=O)(c1ccc(OCCCC(F)(F)F)cc1)C(F)(F)F)c1ccc(OC(F)F)cn1 |r,t:9| |
|---|
| Structure | 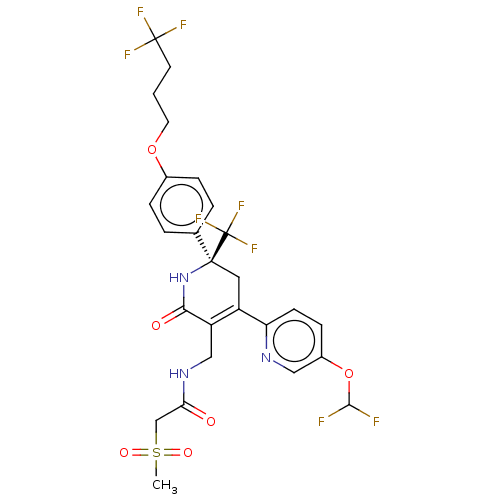
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Nishiura, Y; Kurahashi, K; Ohyabu, N; Sasaki, Y Non-aromatic heterocyclic derivative having MGAT2 inhibitory activity US Patent US10335401 Publication Date 7/2/2019
Nishiura, Y; Kurahashi, K; Ohyabu, N; Sasaki, Y Non-aromatic heterocyclic derivative having MGAT2 inhibitory activity US Patent US10335401 Publication Date 7/2/2019