| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Genome polyprotein |
|---|
| Ligand | BDBM403592 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Ki Determination for Genotypes 1b and 3a NS3 Protease |
|---|
| Ki | 0.040±n/a nM |
|---|
| Citation |  Bjornson, K; Canales, E; Cottell, JJ; Karki, KK; Katana, AA; Kato, D; Kobayashi, T; Link, JO; Martinez, R; Phillips, BW; Pyun, H; Sangi, M; Schrier, AJ; Siegel, D; Taylor, JG; Tran, CV; Trejo Martin, TA; Vivian, RW; Yang, Z; Zablocki, J; Zipfel, S Inhibitors of hepatitis C virus US Patent US10335409 Publication Date 7/2/2019 Bjornson, K; Canales, E; Cottell, JJ; Karki, KK; Katana, AA; Kato, D; Kobayashi, T; Link, JO; Martinez, R; Phillips, BW; Pyun, H; Sangi, M; Schrier, AJ; Siegel, D; Taylor, JG; Tran, CV; Trejo Martin, TA; Vivian, RW; Yang, Z; Zablocki, J; Zipfel, S Inhibitors of hepatitis C virus US Patent US10335409 Publication Date 7/2/2019 |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Genome polyprotein |
|---|
| Name: | Genome polyprotein |
|---|
| Synonyms: | HCV 3a | NS5B | POLG_HCVK3 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 328455.91 |
|---|
| Organism: | Hepatitis C virus genotype 3a (isolate k3a) (HCV) |
|---|
| Description: | Q81495 |
|---|
| Residue: | 3021 |
|---|
| Sequence: | MSTLPKPQRKTKRNTIRRPQDVKFPGGGVIYVGVYVLPRRGPRLGVRATRKTSERSQPRG
RRKPIPKARRSEGRSWAQPGYPWPLYGNEGCGWAGWLLSPRGSRPNWAPNDPRRRSRNLG
KVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRALEDGINFATGNLPGCSFSIFLLA
LFSCLIHPAASLEWRNTSGLYVLTNDCSNSSIVYEADDVILHTPGCIPCVQDGNTSTCWT
PVTPTVAVRYVGATTASIRSHVDLLVGAGTMCSALYVGDMCGPVFLVGQAFTFRPRRHRT
VQTCNCSLYPGHLSGQRMAWDMMMNWSPAVGMVVAHILRLPQTLFDVVAGAHWGIIAGLA
YYSMQGNWAKVAIIMVMFSGVDASTHVTAGQAARNAYGITSLFSVGAKQNLQLINTNGSW
HINRTALNCNESINTGFIAGLFYYHKFNSTGCPQRLSSCKPITFFKQGWGPLTDANITGP
SDDKPYCWHYAPRPCGIVPALNVCGPVYCFTPSPVVVGTTDAKGAPTYTWGANKTDVFLL
ESLRPPSGRWFGCTWMNSTGFVKTCGAPPCNIYGDGRDAQNESDLFCPTDCFRKHPEATY
SRCGAGPWLTPRCLVDYPYRLWHYPCTVNFTLFKVRMFVGGFEHRFTAACNWTRGERCDI
EDRDRSEQHPLLHSTTELAILPCSFTPMPALSTGLIHLHQNIVDVQYLYGIGSGMVGWAL
KWEFVILIFLLLADARVCVALWLILTISQAEAALENLVTLNAVAAAGTHGIGWYLVAFCA
AWYVRGKLVPLVTYSLTGLWSLALLVLLLPQRAYAWSGEDSATLGAGILVLFGFFTLSPW
YKHWIARLIWWNQYTICRCESALHVWVPPLLARGGRDGVILLTSLLYPSLIFDITKLLIA
ALGPLYLIQATITATPYFVRAHVLVRLCMLVRSVMGGKYFQMIILSLADGSNTYLYDHLA
PMQHWAAAGLKDLAVATEPVIFSPMEIKVITWGADTAACGDILCGLPVSARLGREVLLGP
ADDYREMGWRLLAPITAYAQQTRGLLGTIVTSLTGRDKNVVAGEVQVLSTATQTFLGTTV
GGVMWTVYHGAGSRTLAGVKHPALQMYTNVDQDLVGWPAPPGAKSLEPCTCGSADLYLVT
RDADVIPARRRGDSTASLLSPRPLARLKGSSGGPVMCPSGHVAGIFRAAVCTRGVAKALQ
FIPVETLSTQARSPSFSDNSTPPAVPQSYQVGYLHAPTGSGKSTKVPAAYVAQGYNVLVL
NPSVAATLGFGSFMSRAYGIDPNIRTGNRTVTTGAKLTYSTYGKFLAGGGCSGGAYDVII
CDDCHAQDATSILGIGTVLDQAETAGVRLTVLATATPPGSITVPHSNIEEVALGSEGEIP
FYGKAIPIACIKGGRHLIFCHSKKKCDKMASKLRGMGLNAVAYYRGLDVSVIPTTGDVVV
CATDALMTGFTGDFDSVIDCNVAVEQYVDFSLDPTFSIETCTAPQDAVSRSQRRGRTGRG
RLGTYRYVTPGERPSGMFDSVVLCECYDAGCSWYDLQPAETTVRLRAYLSTPGLPVCQDH
LDLWESVFTGLTHIDAHFLSQTKQAGLNFSYLTAYQATVCARAQAPPPSWDETWKCLVRL
KPTLHGPTPLLYRLGPVQNEICLTHPITKYVMACMSADLEVTTSTWVLLGGVLAAVAAYC
LSVGCVVIVGHIELGGKPALVPDKEVLYQQYDEMEECSQARPYIEQAQVIAHQFKEKVLG
LLQRATQQQAVIEPIVVSNWQKLEVLWHKHMWNFVSGIQYLAGLSTLPGNPAVASLMAFT
ASVTSPLTTNQTMFFNILGGWVATHLAGPQASSAFVVSGLAGAAIGGIGLGRVLLDILAG
YGAGVSGALVAFKIMGGEPPTTEDMVNLLPAILSPGALVVGVICAAILRRHVGPGEGPVQ
WMNRLIAFASRGNHVSPAHYVPESDAAARVTALLSSLTVTSLLRRLHQWINEDYPSPCSG
DWLRIIWDWVCSVVSDFKTWLSAKIMPALPGLPFISCQKGYKGVWRGDGVMSTRCPCGAS
IAGHVKNGSMRLAGPRTCANMCHGTFPINEYTTGPSTPCPPPNYTRALWRVAANSYVEVR
RVGDFHYITGATEDGLKCPCQVPATEFFTEVDGVRIHRYAPPCRPLLRDEITFMVGLNSY
AIGSQLPCEPEPDVSVLTSMLRDPSHITAETAARRLARGSPPSEASSSASQLSAPSLKAT
CQTHRPHPDAELVDANLLWRQEMGSNITRVESETKVVILDSFEPLRAETDDAELSAAAEC
FKKPPKYPPALPIWARPDYNPPLLDRWKSPDYVPPTVHGCALPPKGAPPVPPPRRKRTIQ
LDGSNVSAALAALAEKSFPSSKPQEENSSSSGVDTQSSTASKVLPSPGEESDSESCSSMP
PLEGEPGDPDLSCDSWSTVSDSEEQSVVCCSMSYSWTGALITPCSAEEEKLPISPLSNSL
LRHHNLVYSTSSRSASQRQKKVTFDRLQVLDDHYKTALQEVKERASRVKARMLSIEEACA
LVPPHSARSKFGYSAKDVRSLSSKAINQIRSVWEDLLEDTTTPIPTTIMAKNEVFCVDPA
KGGRKAARLIVYPDLGVRVCEKRALYDVIQRLSIETMGSAYGFQYSPRQRVERLLKMWTS
KKTPLGFSYDTRCFDSTVTGQDIRVEEAVYQCCNLEPEPGQAISSLTERLYCGGPMNNSK
GAQCGYLRCRASGVLPTSFGNTITCYIKATAAARAAGLRNPDFLVCGDDLVVVAESDGVD
EDRATLRAFTEAMTRYSAPPGDAPQPTYDLELITSCSSNVSVARDDKGKRYYYLTRDATT
PLARAAWETARHTPVNSWLGSIIMYAPTIWVRMVMMTHFFSILQSQEILDRPLDFEMYGA
TYSVTPLDLPAIIERLHGLSAFSVHSYSPVELNRVAGTLRKLGCPPLRAWRHRARAVRAK
LIAQGGRAKICGLYLFNWAVRTKTKLTPLPAAGQLDLSSWFTVGVGGNDIYHSVSRARTR
YLLLCLLLLTVGVGIFLLPAR
|
|
|
|---|
| BDBM403592 |
|---|
| n/a |
|---|
| Name | BDBM403592 |
|---|
| Synonyms: | Preparation of (1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-1-[(cyclopropylsulfonyl)carbamoyl]-2-(difluoromethyl)cyclopropyl]-9-ethyl-14-methoxy-3,6-dioxo-1,1a,3,4,5,6,9,10,18,19,20,21,22,22a-tetradecahydro-8H-7,10-methanocyclopropa[18,19][1,10,3,6]dioxadiazacyclononadecino[11,12-b]quinoxaline-8-carboxamide | US10335409, Example 2 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C39H52F2N6O9S |
|---|
| Mol. Mass. | 818.927 |
|---|
| SMILES | CC[C@@H]1[C@@H]2CN([C@@H]1C(=O)N[C@@]1(C[C@H]1C(F)F)C(=O)NS(=O)(=O)C1CC1)C(=O)[C@@H](NC(=O)O[C@@H]1C[C@H]1CCCCCc1nc3ccc(OC)cc3nc1O2)C(C)(C)C |r| |
|---|
| Structure | 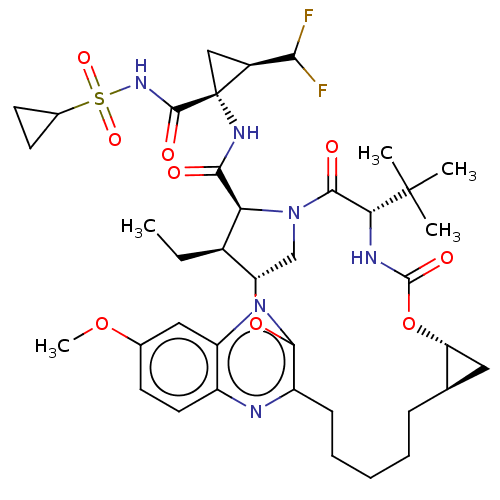
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bjornson, K; Canales, E; Cottell, JJ; Karki, KK; Katana, AA; Kato, D; Kobayashi, T; Link, JO; Martinez, R; Phillips, BW; Pyun, H; Sangi, M; Schrier, AJ; Siegel, D; Taylor, JG; Tran, CV; Trejo Martin, TA; Vivian, RW; Yang, Z; Zablocki, J; Zipfel, S Inhibitors of hepatitis C virus US Patent US10335409 Publication Date 7/2/2019
Bjornson, K; Canales, E; Cottell, JJ; Karki, KK; Katana, AA; Kato, D; Kobayashi, T; Link, JO; Martinez, R; Phillips, BW; Pyun, H; Sangi, M; Schrier, AJ; Siegel, D; Taylor, JG; Tran, CV; Trejo Martin, TA; Vivian, RW; Yang, Z; Zablocki, J; Zipfel, S Inhibitors of hepatitis C virus US Patent US10335409 Publication Date 7/2/2019