| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Eukaryotic translation initiation factor 4 gamma 1 |
|---|
| Ligand | BDBM48833 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation |
|---|
| IC50 | 21900±n/a nM |
|---|
| Citation |  PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID] PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID] |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Eukaryotic translation initiation factor 4 gamma 1 |
|---|
| Name: | Eukaryotic translation initiation factor 4 gamma 1 |
|---|
| Synonyms: | EIF4F | EIF4G | EIF4G1 | EIF4GI | IF4G1_HUMAN | eukaryotic translation initiation factor 4 gamma, 1 isoform 4 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 175455.17 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q04637 |
|---|
| Residue: | 1599 |
|---|
| Sequence: | MNKAPQSTGPPPAPSPGLPQPAFPPGQTAPVVFSTPQATQMNTPSQPRQHFYPSRAQPPS
SAASRVQSAAPARPGPAAHVYPAGSQVMMIPSQISYPASQGAYYIPGQGRSTYVVPTQQY
PVQPGAPGFYPGASPTEFGTYAGAYYPAQGVQQFPTGVAPTPVLMNQPPQIAPKRERKTI
RIRDPNQGGKDITEEIMSGARTASTPTPPQTGGGLEPQANGETPQVAVIVRPDDRSQGAI
IADRPGLPGPEHSPSESQPSSPSPTPSPSPVLEPGSEPNLAVLSIPGDTMTTIQMSVEES
TPISRETGEPYRLSPEPTPLAEPILEVEVTLSKPVPESEFSSSPLQAPTPLASHTVEIHE
PNGMVPSEDLEPEVESSPELAPPPACPSESPVPIAPTAQPEELLNGAPSPPAVDLSPVSE
PEEQAKEVTASMAPPTIPSATPATAPSATSPAQEEEMEEEEEEEEGEAGEAGEAESEKGG
EELLPPESTPIPANLSQNLEAAAATQVAVSVPKRRRKIKELNKKEAVGDLLDAFKEANPA
VPEVENQPPAGSNPGPESEGSGVPPRPEEADETWDSKEDKIHNAENIQPGEQKYEYKSDQ
WKPLNLEEKKRYDREFLLGFQFIFASMQKPEGLPHISDVVLDKANKTPLRPLDPTRLQGI
NCGPDFTPSFANLGRTTLSTRGPPRGGPGGELPRGPAGLGPRRSQQGPRKEPRKIIATVL
MTEDIKLNKAEKAWKPSSKRTAADKDRGEEDADGSKTQDLFRRVRSILNKLTPQMFQQLM
KQVTQLAIDTEERLKGVIDLIFEKAISEPNFSVAYANMCRCLMALKVPTTEKPTVTVNFR
KLLLNRCQKEFEKDKDDDEVFEKKQKEMDEAATAEERGRLKEELEEARDIARRRSLGNIK
FIGELFKLKMLTEAIMHDCVVKLLKNHDEESLECLCRLLTTIGKDLDFEKAKPRMDQYFN
QMEKIIKEKKTSSRIRFMLQDVLDLRGSNWVPRRGDQGPKTIDQIHKEAEMEEHREHIKV
QQLMAKGSDKRRGGPPGPPISRGLPLVDDGGWNTVPISKGSRPIDTSRLTKITKPGSIDS
NNQLFAPGGRLSWGKGSSGGSGAKPSDAASEAARPATSTLNRFSALQQAVPTESTDNRRV
VQRSSLSRERGEKAGDRGDRLERSERGGDRGDRLDRARTPATKRSFSKEVEERSRERPSQ
PEGLRKAASLTEDRDRGRDAVKREAALPPVSPLKAALSEEELEKKSKAIIEEYLHLNDMK
EAVQCVQELASPSLLFIFVRHGVESTLERSAIAREHMGQLLHQLLCAGHLSTAQYYQGLY
EILELAEDMEIDIPHVWLYLAELVTPILQEGGVPMGELFREITKPLRPLGKAASLLLEIL
GLLCKSMGPKKVGTLWREAGLSWKEFLPEGQDIGAFVAEQKVEYTLGEESEAPGQRALPS
EELNRQLEKLLKEGSSNQRVFDWIEANLSEQQIVSNTLVRALMTAVCYSAIIFETPLRVD
VAVLKARAKLLQKYLCDEQKELQALYALQALVVTLEQPPNLLRMFFDALYDEDVVKEDAF
YSWESSKDPAEQQGKGVALKSVTAFFKWLREAEEESDHN
|
|
|
|---|
| BDBM48833 |
|---|
| n/a |
|---|
| Name | BDBM48833 |
|---|
| Synonyms: | (5Z)-3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-nitro-benzylidene)thiazolidine-2,4-quinone | (5Z)-3-(3-chlorophenyl)-5-[(3-methoxy-5-nitro-4-oxidanyl-phenyl)methylidene]-1,3-thiazolidine-2,4-dione | (5Z)-3-(3-chlorophenyl)-5-[(4-hydroxy-3-methoxy-5-nitrophenyl)methylidene]-1,3-thiazolidine-2,4-dione | (5Z)-3-(3-chlorophenyl)-5-[(4-hydroxy-3-methoxy-5-nitrophenyl)methylidene]thiazolidine-2,4-dione | 3-(3-chlorophenyl)-5-(4-hydroxy-3-methoxy-5-nitrobenzylidene)-1,3-thiazolidine-2,4-dione | MLS000676604 | SMR000298496 | cid_1231596 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H11ClN2O6S |
|---|
| Mol. Mass. | 406.797 |
|---|
| SMILES | COc1cc(\C=C2/SC(=O)N(C2=O)c2cccc(Cl)c2)cc(c1O)[N+]([O-])=O |
|---|
| Structure | 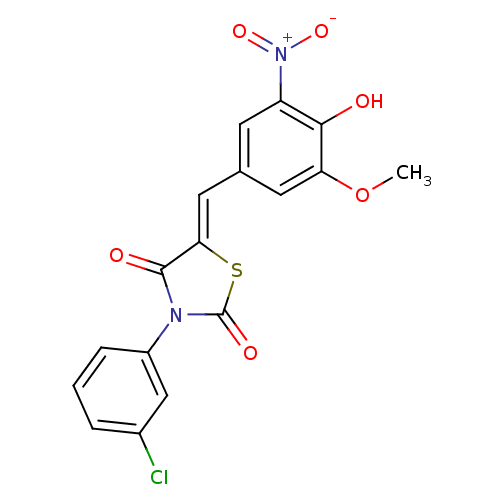
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID]
PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID]