| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Intestinal-type alkaline phosphatase |
|---|
| Ligand | BDBM52332 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Dose Response concentration confirmation of uHTS hits from a small molecule activators of human intestinal alkaline phosphatase via a luminescent assay |
|---|
| EC50 | 12800±n/a nM |
|---|
| Citation |  PubChem, PC Dose Response concentration confirmation of uHTS hits from a small molecule activators of human intestinal alkaline phosphatase via a luminescent assay PubChem Bioassay(2010)[AID] PubChem, PC Dose Response concentration confirmation of uHTS hits from a small molecule activators of human intestinal alkaline phosphatase via a luminescent assay PubChem Bioassay(2010)[AID] |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Intestinal-type alkaline phosphatase |
|---|
| Name: | Intestinal-type alkaline phosphatase |
|---|
| Synonyms: | ALPI | Alkaline phosphatase, intestinal | Intestinal alkaline phosphatase | PPBI_HUMAN |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 56804.94 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_1278525 |
|---|
| Residue: | 528 |
|---|
| Sequence: | MQGPWVLLLLGLRLQLSLGVIPAEEENPAFWNRQAAEALDAAKKLQPIQKVAKNLILFLG
DGLGVPTVTATRILKGQKNGKLGPETPLAMDRFPYLALSKTYNVDRQVPDSAATATAYLC
GVKANFQTIGLSAAARFNQCNTTRGNEVISVMNRAKQAGKSVGVVTTTRVQHASPAGTYA
HTVNRNWYSDADMPASARQEGCQDIATQLISNMDIDVILGGGRKYMFPMGTPDPEYPADA
SQNGIRLDGKNLVQEWLAKHQGAWYVWNRTELMQASLDQSVTHLMGLFEPGDTKYEIHRD
PTLDPSLMEMTEAALRLLSRNPRGFYLFVEGGRIDHGHHEGVAYQALTEAVMFDDAIERA
GQLTSEEDTLTLVTADHSHVFSFGGYTLRGSSIFGLAPSKAQDSKAYTSILYGNGPGYVF
NSGVRPDVNESESGSPDYQQQAAVPLSSETHGGEDVAVFARGPQAHLVHGVQEQSFVAHV
MAFAACLEPYTACDLAPPACTTDAAHPVAASLPLLAGTLLLLGASAAP
|
|
|
|---|
| BDBM52332 |
|---|
| n/a |
|---|
| Name | BDBM52332 |
|---|
| Synonyms: | (E)-3-[5-(4-Chloro-phenyl)-furan-2-yl]-2-(5-methyl-4H-[1,2,4]triazol-3-ylsulfanyl)-acrylic acid | (E)-3-[5-(4-chlorophenyl)-2-furanyl]-2-[(5-methyl-1H-1,2,4-triazol-3-yl)thio]-2-propenoic acid | (E)-3-[5-(4-chlorophenyl)-2-furyl]-2-[(5-methyl-1H-1,2,4-triazol-3-yl)thio]acrylic acid | (E)-3-[5-(4-chlorophenyl)furan-2-yl]-2-[(5-methyl-1H-1,2,4-triazol-3-yl)sulfanyl]prop-2-enoic acid | MLS000566093 | SMR000145538 | cid_1399593 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H12ClN3O3S |
|---|
| Mol. Mass. | 361.803 |
|---|
| SMILES | Cc1nnc(SC(=Cc2ccc(o2)-c2ccc(Cl)cc2)C(O)=O)[nH]1 |w:7.7| |
|---|
| Structure | 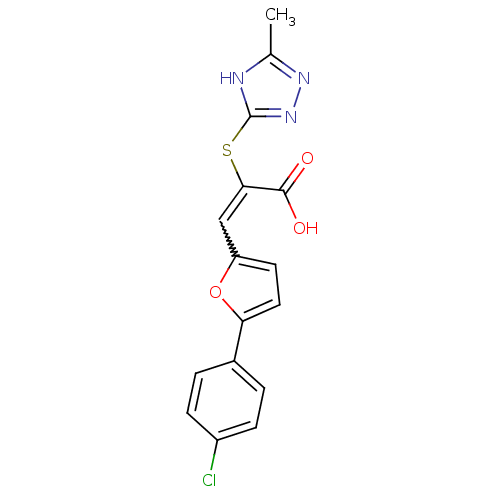
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 PubChem, PC Dose Response concentration confirmation of uHTS hits from a small molecule activators of human intestinal alkaline phosphatase via a luminescent assay PubChem Bioassay(2010)[AID]
PubChem, PC Dose Response concentration confirmation of uHTS hits from a small molecule activators of human intestinal alkaline phosphatase via a luminescent assay PubChem Bioassay(2010)[AID]