| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phospholipase A2 |
|---|
| Ligand | BDBM50648 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Dose response counterscreen of uHTS hits for ATG4B inhibitors in a Phospholipase A2 assay |
|---|
| IC50 | >80000±n/a nM |
|---|
| Citation |  PubChem, PC Dose response counterscreen of uHTS hits for ATG4B inhibitors in a Phospholipase A2 assay PubChem Bioassay(2011)[AID] PubChem, PC Dose response counterscreen of uHTS hits for ATG4B inhibitors in a Phospholipase A2 assay PubChem Bioassay(2011)[AID] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phospholipase A2 |
|---|
| Name: | Phospholipase A2 |
|---|
| Synonyms: | Group IB phospholipase A2 | PA21B_HUMAN | PLA2 | PLA2A | PLA2G1B | PPLA2 | Phosphatidylcholine 2-acylhydrolase 1B | Phospholipase A2 (PLA2) | Phospholipase A2 group 1B | phospholipase A2 precursor |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 16364.13 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P04054 |
|---|
| Residue: | 148 |
|---|
| Sequence: | MKLLVLAVLLTVAAADSGISPRAVWQFRKMIKCVIPGSDPFLEYNNYGCYCGLGGSGTPV
DELDKCCQTHDNCYDQAKKLDSCKFLLDNPYTHTYSYSCSGSAITCSSKNKECEAFICNC
DRNAAICFSKAPYNKAHKNLDTKKYCQS
|
|
|
|---|
| BDBM50648 |
|---|
| n/a |
|---|
| Name | BDBM50648 |
|---|
| Synonyms: | MLS000762704 | N-[(5Z)-5-[(E)-3-(2-furanyl)prop-2-enylidene]-4-oxo-2-sulfanylidene-3-thiazolidinyl]-4-pyridinecarboxamide | N-[(5Z)-5-[(E)-3-(2-furyl)prop-2-enylidene]-4-keto-2-thioxo-thiazolidin-3-yl]isonicotinamide | N-[(5Z)-5-[(E)-3-(furan-2-yl)prop-2-enylidene]-4-oxidanylidene-2-sulfanylidene-1,3-thiazolidin-3-yl]pyridine-4-carboxamide | N-[(5Z)-5-[(E)-3-(furan-2-yl)prop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]pyridine-4-carboxamide | N-{5-[(E)-3-Furan-2-yl-prop-2-en-(Z)-ylidene]-4-oxo-2-thioxo-thiazolidin-3-yl}-isonicotinamide | SMR000438207 | cid_5922856 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H11N3O3S2 |
|---|
| Mol. Mass. | 357.407 |
|---|
| SMILES | O=C(NN1C(=S)S\C(=C/C=C/c2ccco2)C1=O)c1ccncc1 |
|---|
| Structure | 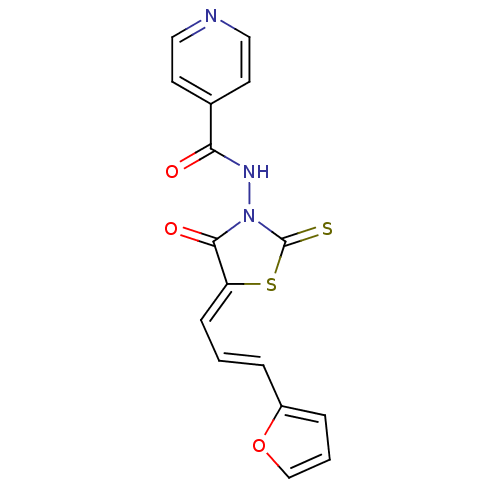
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 PubChem, PC Dose response counterscreen of uHTS hits for ATG4B inhibitors in a Phospholipase A2 assay PubChem Bioassay(2011)[AID]
PubChem, PC Dose response counterscreen of uHTS hits for ATG4B inhibitors in a Phospholipase A2 assay PubChem Bioassay(2011)[AID]